Overview of the study design
Introduction
You get to the Overview of the study design page, in one of the following ways:
- When clicking the link from the study summary page:
- After initiating a design, either by creating a new design from scratch, or by importing an existing version. See Initiating a design / Importing a new design version / Adding a new empty design version.
Overview of the study design
The Overview of study design page consists of the following main areas:
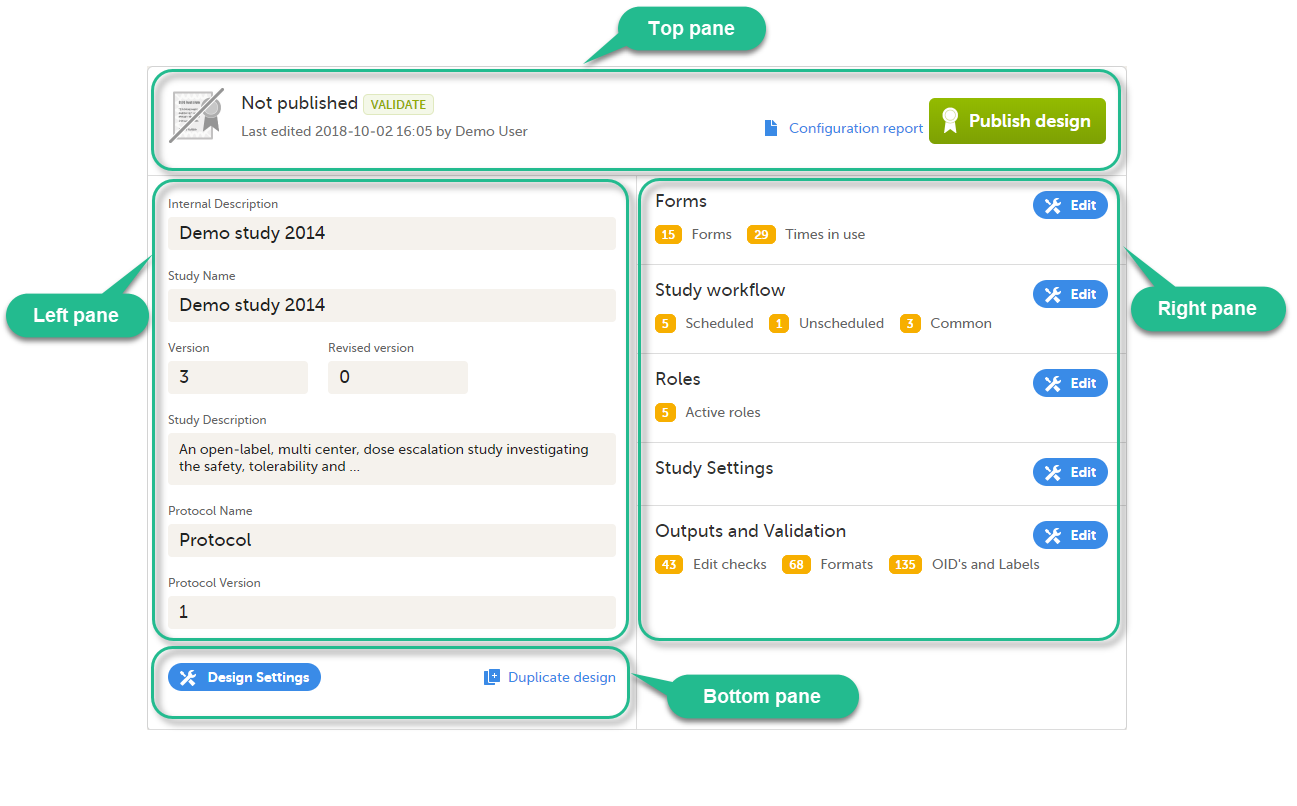
- Top pane - provides information on whether the design version is published or not, when it was last edited and by whom, as well as links for:
- Validate the design - for details, see Validating a study design.
- Configuration report - for details, see Configuration report.
- Publish design - for details, see Publishing a study design.
- Left pane - descriptive information of the study design
- Right pane - links to view/edit:
- Forms - here you configure the forms that will be used within the study design. For details, see Creating and editing forms.
- Study workflow - here you can set up the events in the study, and populate the events with activities and forms. For details, see Study workflow.
- Roles - here you configure the clinic roles and their permissions within the study. For details, see Configuring roles.
- Study settings - for details, see:
- Selection View Settings - here you can configure the information to be displayed on the subject card.
- Subject Id Generation Settings - here you can set up the format for the Subject ID, used to identify a subject within the system.
- SDV Settings - the Source Data Verification (SDV) setting enables you to choose what forms and items to require SDV in your study.
- Miscellaneous
- Alerts - here you can set up alerts in your study to notify users about important occurrences in the data.
- Subject Status - here you define the statuses of a subject, that will be used for calculating the Metrics displayed in Clinic.
- Setting up the randomization - here you configure randomization, if used within your study.
- eLearning settings - here you configure the eLearning curriculums that should be available for the clinic users in your study.
- Outputs and Validation - this section summarizes some of the item settings that performed in Viedoc Designer and provides a better overview and an easier way to update those. For details, see Outputs and validation.
- Bottom pane - links for:
- Design settings - directs you to the Design settings page, where you can export, lock or delete the design. For details, see Exporting/Locking/deleting a study design.
- Duplicate design - for creating a new version/revision of the design. For details, see Duplicate a design - versions and revisions.
