Viedoc study configuration management
Introduction
The configuration of a study in Viedoc consists of two types of settings:
- Non version-controlled settings - settings that are configured by the Study Manager in the Viedoc Admin application and that are effective immediately. These settings are common to the study at any given point in time.
They are described in detail in the lesson General study settings. - Version-controlled settings - settings that are configured by the Designer in Viedoc Designer, but not effective until assigned to site(s) by the Study Manager in Viedoc Admin.
This lesson focuses on the configuration type that holds most of the study configuration, that is version-controlled settings.
Configuration overview
Study design version numbers
Version-controlled settings are contained in a “design” and are identified by a version number. Study design version numbers are unique within a study. If there exist five study design versions, all originating from the same design, and a new design is created from scratch within the same study, it will have version 6.
Study design version numbers are accompanied with a revision number. For example, “1.0” means that this is version 1 and that it has not been revised, since the revision part of the version is 0. Revisions are explained in Revision of study design version.
Assignment of study design to site(s)
Study designs are assigned on site level. Work on a site cannot start before a study design is assigned to that site, as there is no study configuration associated with that site.
When the Designer has finished setting up a study design in Viedoc Designer, he/she has to publish the study design, so that it becomes available to the Study Manager in Viedoc Admin.
The Study Manager then chooses to assign the study design to one or several study sites. This step is accompanied with selecting an effective starting time for the study design on the selected study sites.
There can be more than one design version assigned to a site.
Version "burn-in"
Versions are burnt in at event level, based on the date of first data entry.
The applicable design version for an event is determined by comparing the event date to the effectiveness period of the study design version(s) assigned to the site. When an instance of an event is started, the study design version is burnt into it, indefinitely. All forms belonging to this event will then inherit that same study design version.
When the version has been burnt-in, the forms within the event always get their settings, structure and lay-out read from that same study design version, even if the event date, or design effectiveness periods, have changed so that a different study design version is available.
Event dates
In Viedoc, there are four different types of events, and the study design version is burnt in as follows:
- Study start event - typically one simple form containing patient identification data. The study start event has no manual event date entry. Thus, even if it only contains one form and this form contains something called a “start date”, it will not be used as event date. The event date of this type of event is always the time of event initialization, that is, when the event instance is entered for the first time.
- Scheduled events - visits scheduled according to the protocol. These require a date to be input when they are started/initiated. For these events this is the event date, and the study design version will burn in when the event is first started/initiated. If the event date is changed there will be no new burn-in. However, until the first form of the event is entered, the event can be stopped/uninitiated and this is then treated as a reset of the event and a new burn-in will occur when the event is again started/initiated.
- Unscheduled events - additional, on-demand visits. These require a date to be input when they are started/initiated. For these events this is the event date, and the study design version will burn in when the event is first started/initiated. If the date is changed there will be no new burn-in. However, until the first form of the event is entered, the event can be stopped/uninitiated. This is then treated as a reset of the event and a new burn-in will occur when the event is again started/initiated.
- Common events - events occurring separately or parallel to the workflow, for example concomitant medication, adverse events, dose adjustments, daily compliance reporting. These have no manual date entry. Thus, even if they only contain one form and this form contains something called a “start date”, it will not be used as event date. The event date of these types of events are always the time of event initialization, that is, when the event instance is entered for the first time.
Notes!
- The above applies even for the events that are using the "automatic event date" functionality, that is, if the event is configured to use an event date based either on the date of first data entry or on a date item.
- Events that are added using the automatic event date functionaility will burn-in the current effective design.
For more details on the automatic event date settings, see the Study workflow lesson.
Multiple versions over time
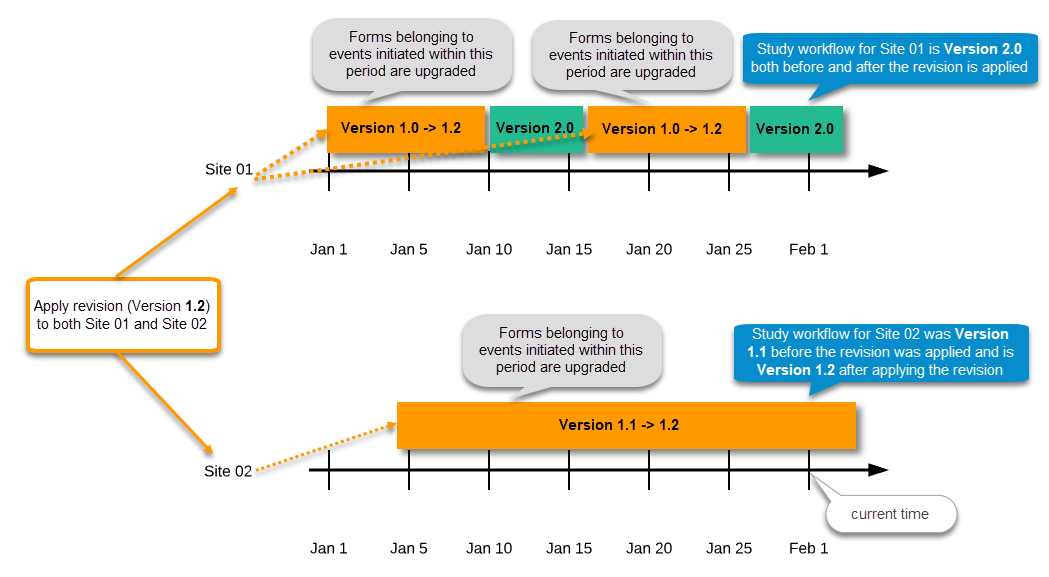
During the course of a study, multiple versions of the study design can be assigned to a site, with different periods of effectiveness. For example, Site 01 could have Version 1 as the effective study design version during January 1st – January 15th and Version 2 as effective study design version after January 15th, whereas Site 02 has Version 2 as the only effective study design version starting January 5th, as illustrated in the image:
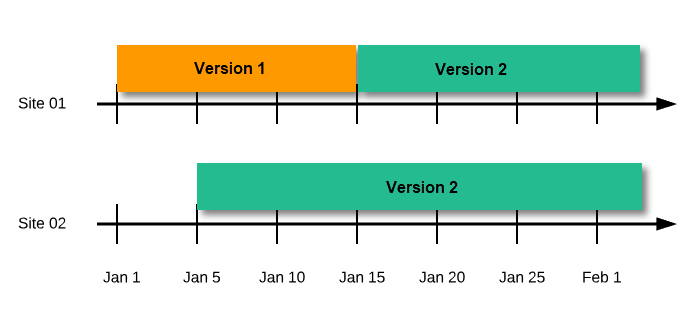
There is no end date for the effectiveness periods of study design versions. If a design is applied on January 1st, this is the effective version until a new version with a later start date is encountered, independently of when it was assigned.
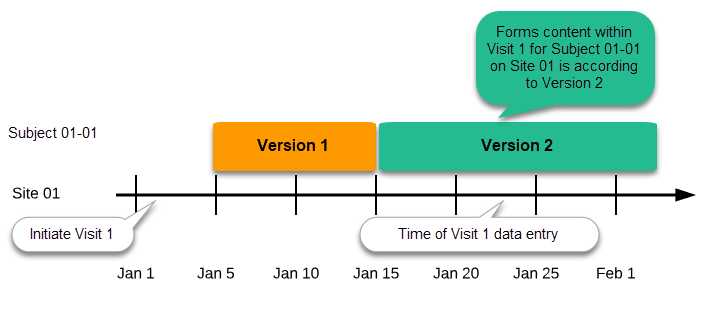
Important! The periods of effectiveness of a study design version are connected to the event timing of the first data entry and not to the current time at system usage.
For example, in the below image, if:
- Version 1 is effective between January 1st - January 15th for Site 01.
- Version 2 is effective starting with January 16th for Site 01.
- Event 1 is initiated on January 2nd for Subject 01-01 on Site 01.
...then:
- Form content of Event 1 for Subject 01-01 on Site 01 will always be according to Version 1 (because Event 1 was initiated within the validity period of Version 1), regardless of the time of data entry:

Event created before starting point of any version
If an event is initiated with a date when no design version is in effect, the version effective at current time of system usage will be used:
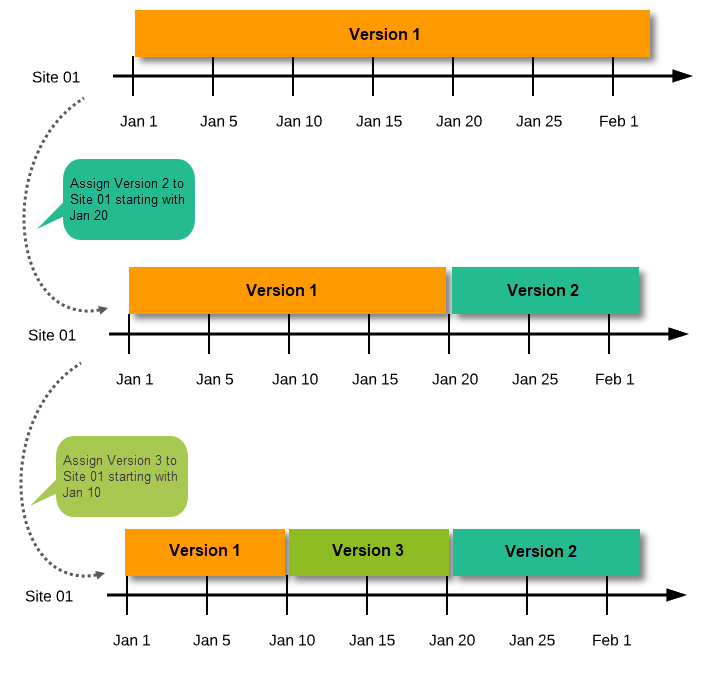
New version with same timing as current version
A new version can be assigned with the same timing as the currently assigned version and will then replace the currently assigned version, except for already entered data (due to the version “burn-in”, as described in Version burn-in).
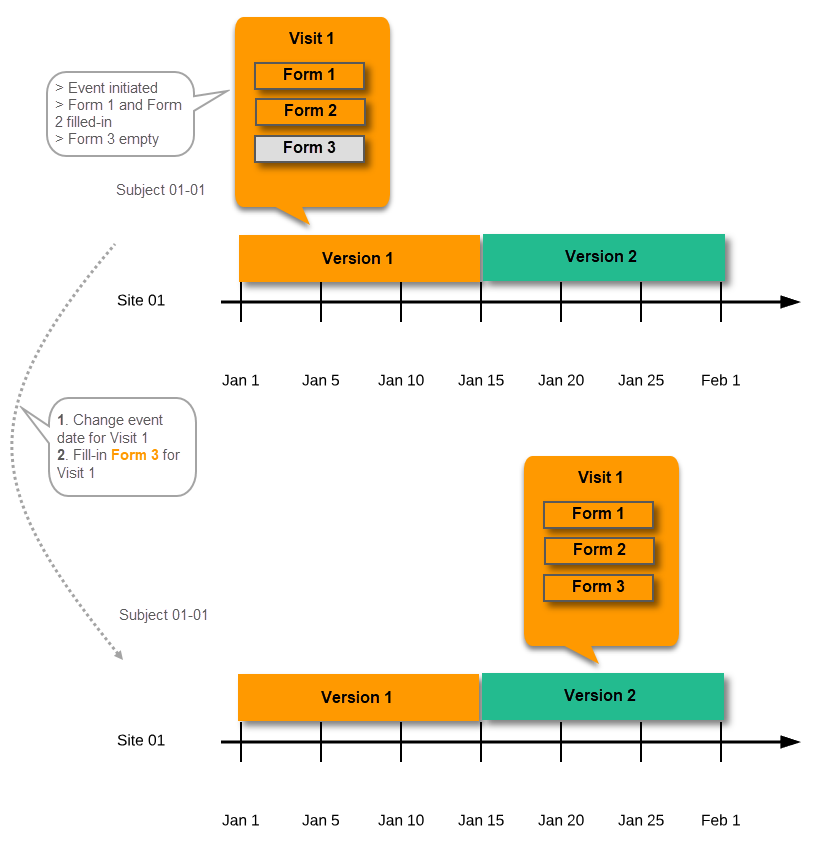
For example, if we have Version 1 assigned to Site 01 starting at Jan 1, and we have the following events:
- Event 1 - initiated and containing 3 forms:
- Form 1 and Form 2 filled in
- Form 3 empty
- Event 2 - not initiated
...and we assign Version 2 to Site 01 starting at Jan 1, and then:
- fill in Form 3 for Event 1 - this will have also Version 1, as this was burnt-in at the time when Event 1 was initiated.
- initiate Event 2 and fill in Form 1, this will have Version 2 (as well as all the other forms within Event 2)
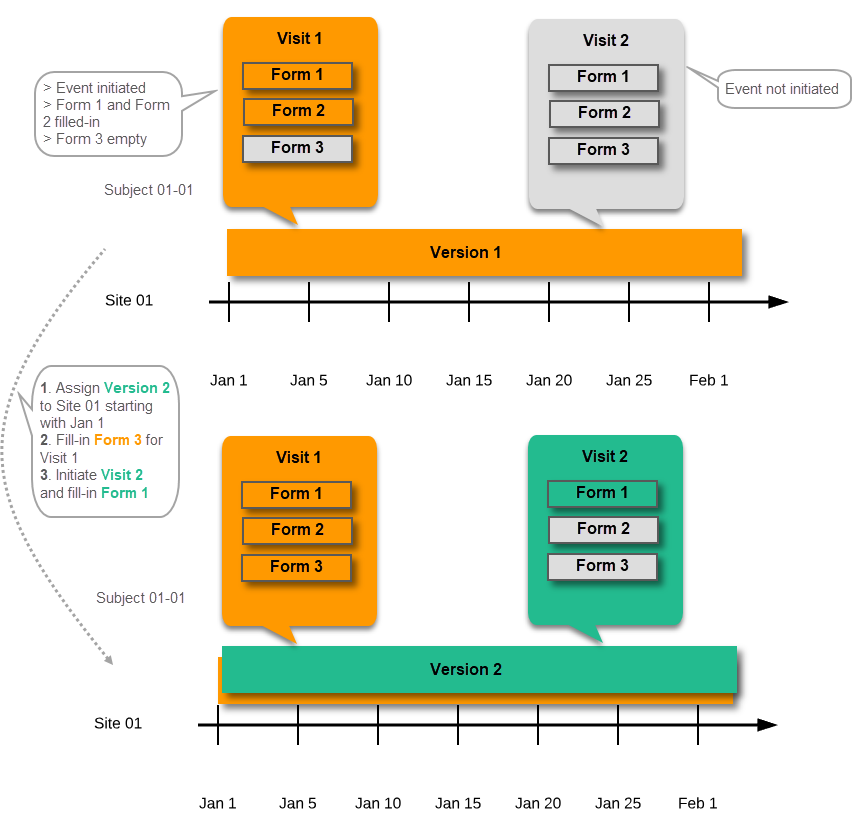
Event date changed after it was initiated
In case the event date is changed after it was initiated, to a date when another version is applicable, the version for that event does not change, as it was burnt-in at the date when the event was initiated (see Version burn-in):
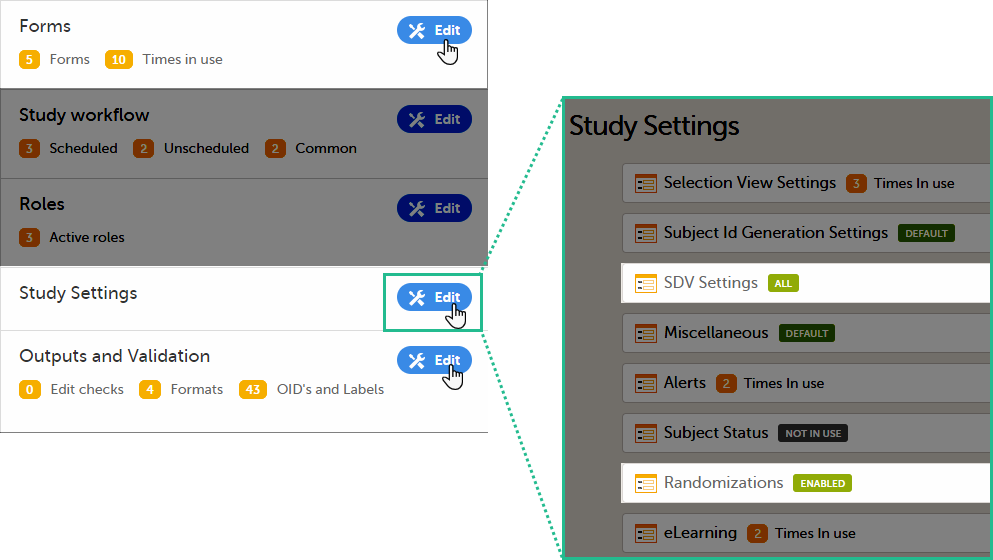
Settings read from the study design version burnt-into the event
The following settings are always read from the study design version that is burnt-into the event:
- Forms
- Study settings
- Source Data Verification (SDV) settings
- Randomizations
- Output and Validation
- Automatic data validation (“Edit checks”)
- Output formats
- Output ID:s and labels
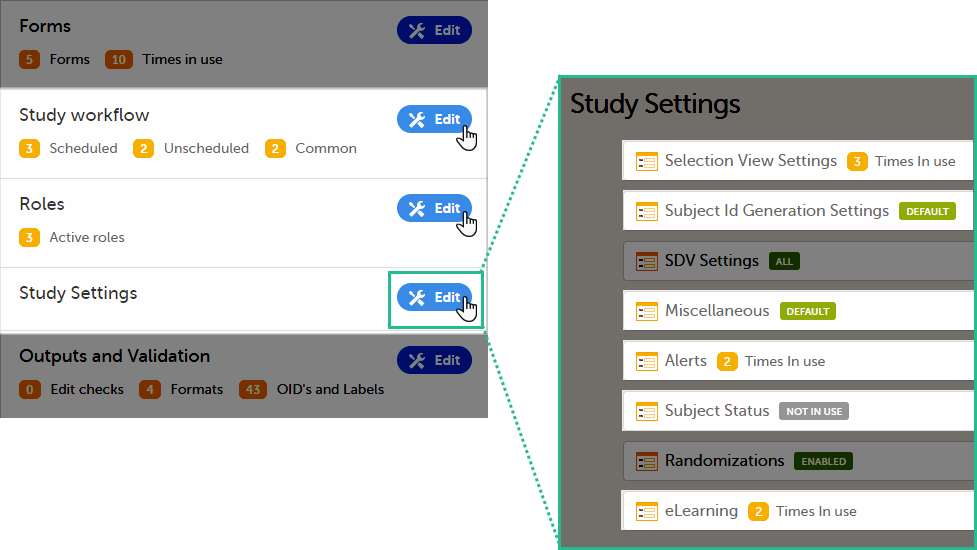
Settings read from current effective design
We call "current effective design" the study design version that is effective at the current time of system usage.
Settings that are not directly related to data collection structure, as well as settings that are common on the site level, are read from the study design version that is effective at the time of system usage (that is, “time right now”). These settings are:
- Study workflow
- Study start event
- Scheduled events
- Unscheduled events
- Common events
- Roles
- Study settings
- Layout of subject card and subject selection page
- Subject Id format
- Miscellaneous
- Alerts
- Subject status
- eLearning
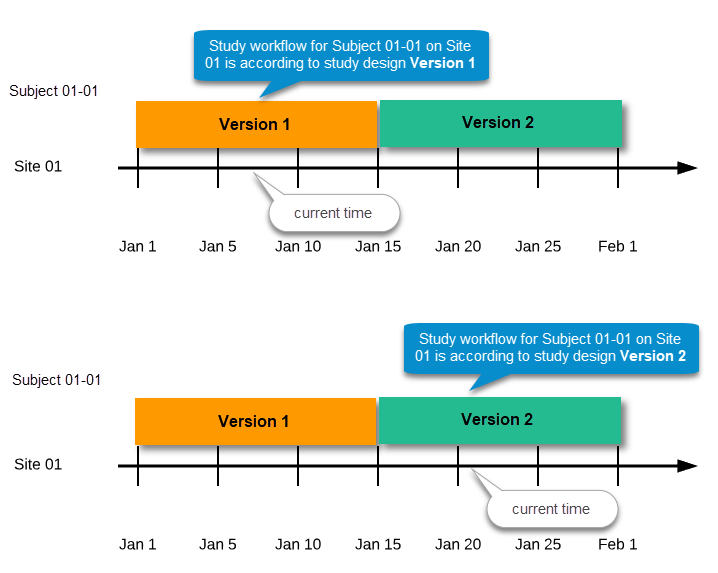
This means that the study workflow for a subject can change as time passes and a new design version becomes effective for the site the subject belongs to:
Revisions of study design version
If there is a need to correct something in a study design version that is already assigned, and in particular if it has already been used to enter data (as that version is then burnt in and cannot be replaced by assigning a new version with the same time frame of validity), the study design version has to be revised.
The following settings can be revised as part of a revision of a study design version:
- Forms *
- Study workflow *
- Roles
- Output and Validation
The latest effective design for each site will be used to define the permissions that will apply to each role.
* Note! The study design IDs (Event IDs, Activity IDs, Form IDs, Item group IDs and Item IDs), item dictionary (“choice”) codes and any items involved in randomization cannot be changed. An exception is that if an item needs to be moved to another item group, the ID can and must be changed as this will be treated as deleting an item and adding it again.
Once a study design version is revised, the revision part of the version number (initially 0) is incremented. For example, if study design version 1.0 is revised, it will receive the version number 1.1. When a revision of a study design version is published, it replaces its predecessor in terms of site assignment. For example, if a Study Manager wants to assign version 1 to a site, and this version now has a revision, version 1.1 will be available for assignment and not 1.0.
Additionally, only the latest revision of each study design version can be used as starting point for additional revision. For example, if we want to revise study design version 1, that has already been revised to version 1.1, we can only select 1.1 as starting point of the revision and not 1.0.
If forms that were previously part of an event in the workflow are now removed, already initiated forms are not touched. From a study workflow point-of-view they are now orphan forms, but from a user point-of-view there is no real difference to the appearance, as they stay as is on the event that they were previously part of.
Applying a revised study design version to a site
Application of a revised study design version is used to upgrade forms that are already burnt-in, with a predecessor of the study design version in terms of revisions, to the latest revision of the study design. Applying a revision is different from assigning study design versions, as assigning study design versions only affects forms belonging to events that have not been initiated yet.
When a revised version of a study design version is applied to a site, that revision will (eventually, after necessary site confirmations, see Changes in a revision that affect data integrity below) replace the version it is revising, including the base version and all previous revisions made to the version (as illustrated in the example in the image below, where 1.2 replaces both 1.0 and 1.1). Thus, effective period is not changed.
There are two parallel tracks being followed when a revised study design is applied, depending on whether the data integrity is affected by the changes in a revision or not, as described in the following subsections.
Note! It is recommended that you use the design revision impact analysis before you apply any revision. For more information, see Design revision impact analysis.
Note! You can NOT apply an earlier revision if a later revision has already been applied to another site. This applies to both demo sites and production sites. For example, if version 1.2 has already been applied to a site with user acceptance testing (UAT) underway, then version 1.1 cannot be applied to a production site. It is only possible to apply version 1.2.
Changes in a revision that do not affect data integrity
A revision with changes that do not affect data integrity is applied without confirmation by the site staff. For all form instances in which form data is not affected by the revision, the revision is processed immediately.
Changes within a revision that do not affect data integrity:
- Forms – updated settings on:
- Required/optional
- Data checks
- Min and max length
- Number of decimal digits and/or number of decimals for numeric data type
- Output IDs and labels
- Study workflow – actual workflow changes
Note! If these are the only changes made, this will be the same as assigning the study design to the site, as workflow is always read from the version effective at time of system usage as pointed out in Settings read from current effective design.- Addition/deletion of events
- Addition/deletion of activities/forms on existing event
- Roles
- Output and Validation
Any discrepancies no longer valid are closed and any new discrepancies will be flagged.
The forms that are locked (by Monitor, Data Manager, or any user who has data lock permission) will be upgraded, as no data will be touched by these types of changes.
Form signature and review status will not be affected by these kind of updates.
Changes in a revision that affect data integrity
Applying a revision with changes that potentially do affect data integrity requires confirmation by the site staff. Before the Study Manager can apply a revised study design, a mandatory information text has to be entered that will be used to notify the site staff about the changes (see the complete workflow below in Workflow - Revision of an existing version).
Changes that potentially do affect data integrity:
- Forms – addition/deletion of items and changes to:
- Name of form
- Item labels, including static text items
- Item and item group position and input field size
- Measurement units
- Dictionary (“choice”) labels
- Instruction texts
- Visibility conditions
Note! Changes of the role visibility conditions do not require site approval. - Function and default value expressions
- Study Workflow
- Visibility conditions affecting form contents
- Event date settings
Note! Changes of the event date(s) as a result of changing the "automatic event date" settings do not require site approval. For details on the automatic event date settings, see the Study workflow lesson.
A flag will be put on the form instance indicating that there is an upgrade pending. Until the upgrade is confirmed by site personnel (see Site confirmation of version upgrade), the form will remain in its original version.
Site confirmation of version upgrade to revised version
When a revised study design is applied to a site, all forms pending an upgrade (where data integrity is potentially affected) are marked by a red flag, and a notification for the site is displayed on the Messages pane on the study start page.
The notification is accompanied with a standard text informing the site about the upgrade action and confirmation prompt that has to be electronically signed. This action can be performed by anyone at the site with data edit permission. See also the lesson in Viedoc Clinic User Guide - Approving eCRF changes.
When signed, all forms pending upgrade (listed in Changes in a revision that affect data integrity) will be upgraded to the revised version of the study design. This is a background activity that can take some time if there are considerable amounts of forms to be upgraded.
For the subjects that are being edited by clinic users during the upgrade process, the upgrade will stay pending until the respective subjects are released.
Optionally, forms can be upgraded manually, one-by-one, by the site. This is performed by navigating to the forms affected, opening them for editing and re-saving them. When the form is opened for editing, it will be shown using the new upgraded design with all data filled in.
A recommendation to the site could be to manually upgrade a few forms to fully understand the potential impact of the upgrade and then upgrade the rest using the batch approval feature.
|
Important! The upgrade is not performed for:
If performing batch approval and forms affected by the upgrade are skipped, as a result of one of the above mentioned scenarios, a new message will be displayed on the Message page. The changes can then be approved after a user with permission unlocks the locked forms. |
Forms upgraded to a new design revision lose any existing signature and review flags (clinical review, data review). The SDV flag is lost on item level.
If a particular item, that did not require SDV, is affected by the upgrade (that is, edited in the design, see Changes in a revision that affect data integrity), the form-level SDV flag will be kept. That means an upgrade of a form can lead to losing existing signature and review flags (clinical review, data review), but keeping the SDV flag.
If a particular item, that was previously source data verified, is affected by the upgrade (that is, edited in the design, see Changes in a revision that affect data integrity), it is no longer flagged as having been source-data verified. The form-level SDV flag is lost if any item in the form lost its SDV flag. The form-level SDV flag is also lost if an item is removed from a form as part of the upgrade.
Configuration workflow
New study - first study design version
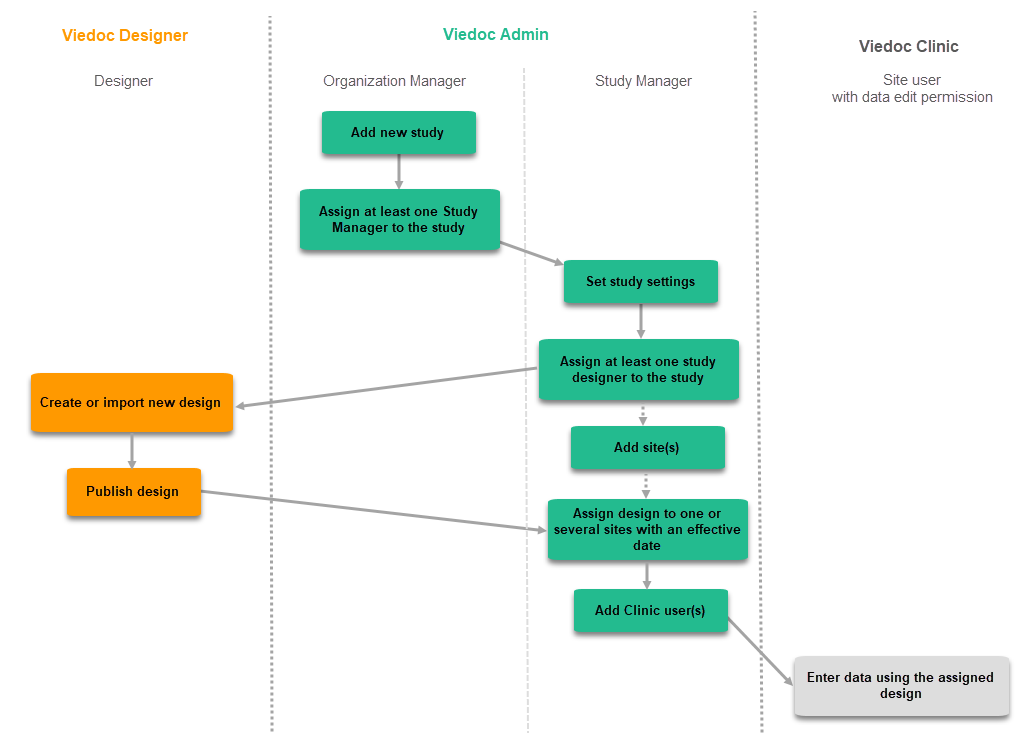
The following steps have to be performed in Viedoc when creating and configuring the study for the first time:
- In Viedoc Admin, the Organization Manager creates the new study and appoints a Study Manager.
- In Viedoc Admin, the Study Manager sets the non version-controlled common settings and appoints a Designer.
- In Viedoc Designer, the Designer creates the first version of the version-controlled settings and makes it available to the Study Manager by publishing the design to Viedoc Admin.
- In Viedoc Admin, the Study Manager creates site(s) and assigns the first version to the site(s)
- The site user can start entering data in Viedoc Clinic, using the assigned design.
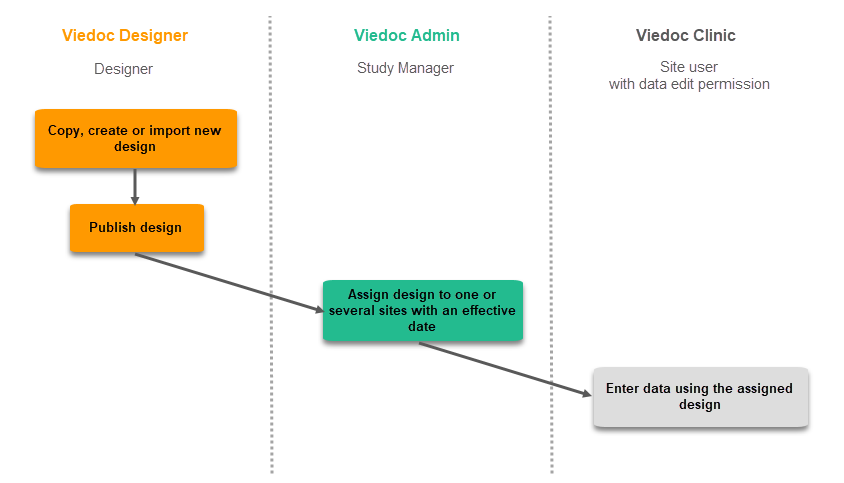
Subsequent versions
The workflow for creating a new design version starting from an existing version of the study design, to implement a protocol amendment and assign it to one or several sites, is the following:
- In Viedoc Designer, the Designer makes a full copy of the existing version of the study design.
- In Viedoc Designer, the Designer makes the additional settings required by the protocol amendment and then publishes the design, which then becomes available in Viedoc Admin.
- In Viedoc Admin, the Study Manager assigns the new version to one or several sites that should have the amendment. For instructions see Assigning a study design.
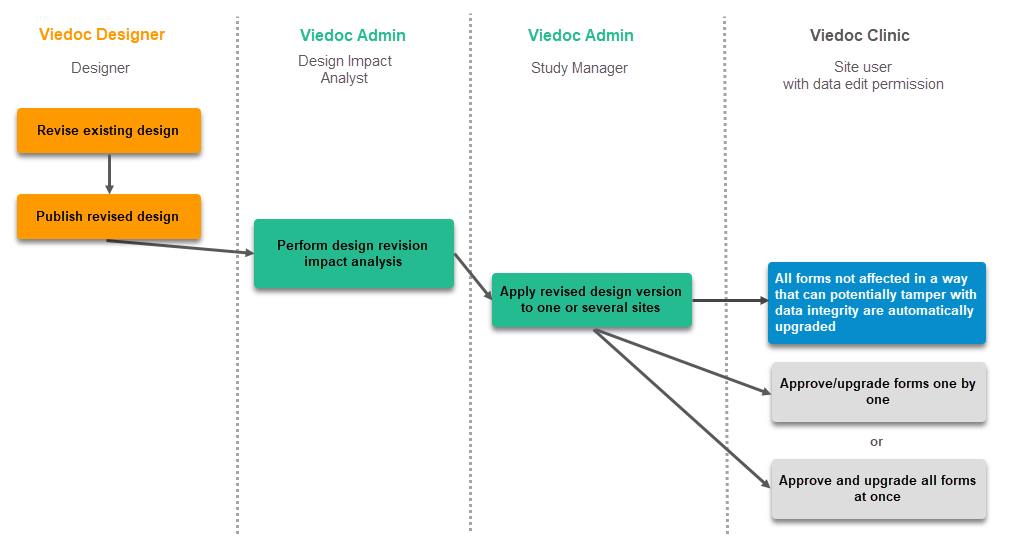
Revision of existing version
The workflow for revising an existing study design version and applying it to one particular site is as following:
- In Viedoc Designer, the Designer makes a revision of an existing version.
- In Viedoc Designer, the Designer makes the additional settings and then publishes the design that becomes available in Viedoc Admin.
- In Viedoc Admin, the Design Impact Analyst performs the revision impact analysis. For more information, see Design revision impact analysis.
- In Viedoc Admin, the Study Manager applies the revised version to one or several sites that should have the revision. For instructions see Assigning a study design.
Important! If a new revision is applied before previous one(s) are approved by a site user, then the approval will upgrade affected forms to the latest revision, regardless of which of the upgrades the site user approves. For information on site approval see Approving eCRF changes in Viedoc Clinic User Guide.
Note! An upgrade message is displayed for the site under the Messages pane on the study start page, even for the revisions with changes that do not affect data integrity, that is, when the forms are automatically upgraded.
Configuration management
If an iterative approach is used during development of a configuration, there will be many versions created as part of the workflow: [ setup --> test --> correct --> test --> setup --> test --> correct --> test --> … ]. It is still advisable to revise existing versions (instead of creating new versions) whenever possible to keep the number of versions to a minimum, as this will make the study design repository less cluttered and more easy to manage.
In Viedoc Designer
In Viedoc Designer, the following actions related to the study design can be performed, as described in the respective lessons:
- Initiating a study design - describes how to initiate a design, either by adding a new empty version or by importing one.
- Validating a study design
- Publishing a study design - describes how to publish and unpublish a design.
- Duplicating a design - describes how to either create a new version by copying an existing version, or revise an existing version.
- Exporting/Locking/Deleting a study design
In Viedoc Admin
In Viedoc Admin, you can see the current effective design for each site, assign a study design version to one or several sites, and apply a revised version of a study design to one or several sites. All these are described in detail in Assigning a study design.
