Duplicating a design - versions and revisions
Introduction
The settings within the study design are version controlled and identified by the version number. Study design version numbers are unique within a study, for example if there are five study design versions, all originating from the same design, and a new design is created from scratch within the same study, it will have version 6.
Study design version numbers are accompanied with a revision number, that is “1.0” means version 1 and that it has not been revised as the revision part of the version is 0. In a similar way, "1.6" means revision 6 of version 1.
Study design versions and revisions are explained in detail in Viedoc study configuration management.
After a design version was published, changes to the study design can be performed by clicking Duplicate design in the study design overview page.

There are two different ways to perform changes on an existing version of the study design:
- Fully customizable version (1) - creates a new version, for example if the current version is 1.4, this will make a copy of that and get version 2.0, as described in detail in Viedoc study configuration management.
- Revised version (2) - if the current version is 1.4, this will make a copy but instead create a revision with version 1.5. as described in detail in Viedoc study configuration management.
Note! If the Revised version option is not available in the Duplicate design pop-up, it can be one of two reasons:- This revision has not yet been assigned or applied to any site. You should then continue working with the existing revision. To do that, you first need to unpublish the design and then unlock it (see Publishing a study design). When this is done you can continue working with the revised version.
- Viedoc Designer has not yet registered that the revision has been assigned or applied. In this case, refreshing the page should resolve the problem.
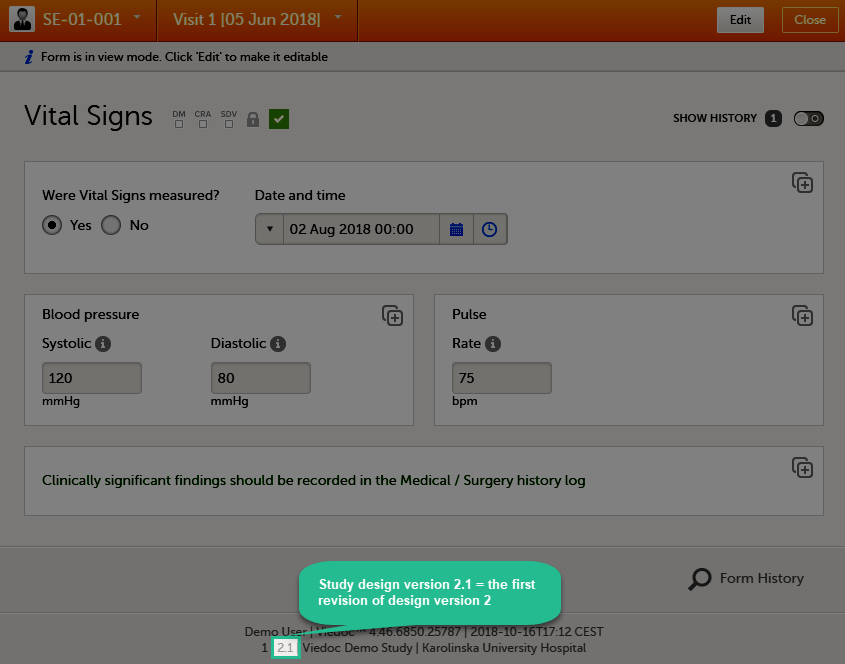
The current version and revision number of the study design are displayed on the study design overview page:

The version and revision number of the study design used to initiate the form instance is displayed in the form footer in Viedoc Clinic:
Create a new version or revise an existing one?
In an ideal situation when the version of the study design that is assigned at study start is perfect, new versions should only be used when there is an actual need to have different versions of the “study” over time or on different sites, that is due to protocol amendments, or other legit differences like data collection restrictions in different countries.
The general rule is therefore:
- Revise an existing version if you need to make changes that are applicable to already entered data, for example correct an error or complete an incomplete setup. Revisions are explained in detail in Viedoc study configuration management.
- Create a new study design version if you need to create a new study design track with changes that are not applicable to already existing data. Versions are explained in detail in Viedoc study configuration management.
However, some changes are not allowed as part of a revision and requires creation of new versions more often than the rule suggests. See Viedoc study configuration management lesson for a detailed list on which changes are allowed as a part of a revision.
