Importing a new design version
Introduction
This lesson describes the steps to be performed when you already have a couple of design versions and want to import a new design version.
The case of importing a new design at the very beginning, when no design version exists for the study, is described in the lesson Initiating a design.
Importing a new design version
The format supported for importing a design is Clinical Data Interchange Standards Consortium (CDISC) Operational Data Model (ODM) with or without CDISC Study/Trial Design Model and Viedoc extensions – which means that it is possible to import study designs from manually created configurations, or from configurations generated in other systems, as long as they are CDISC compliant.
To import a new design version to your study:
| 1 |
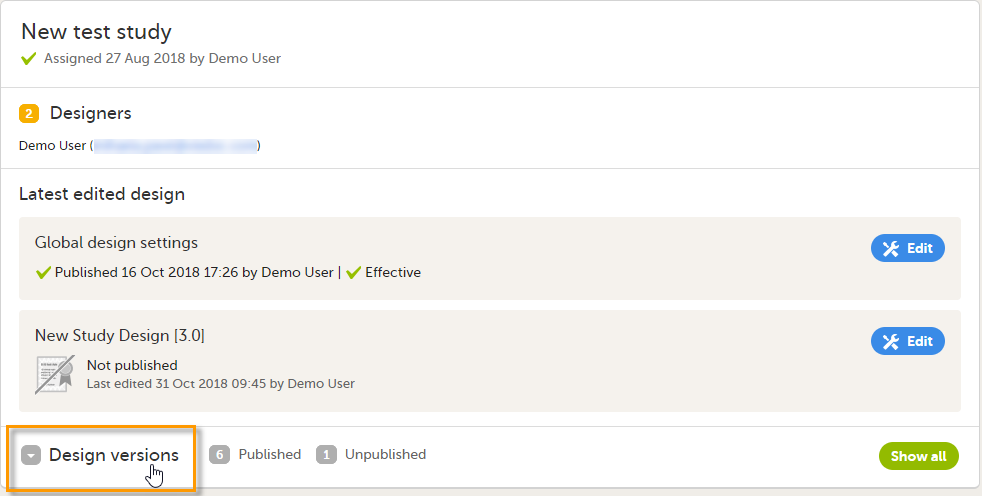
In Viedoc Designer, go to your study and click Design versions: A list of all existing design versions is displayed: |
| 2 |
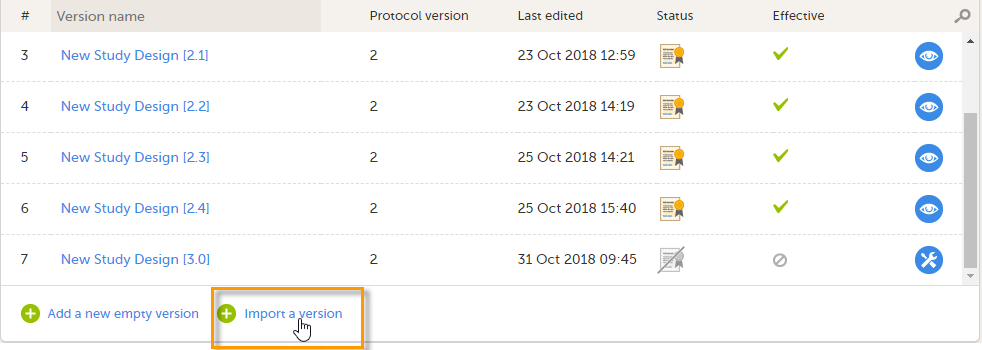
In the bottom of the list, click Import a version. The Import design pop-up opens: |
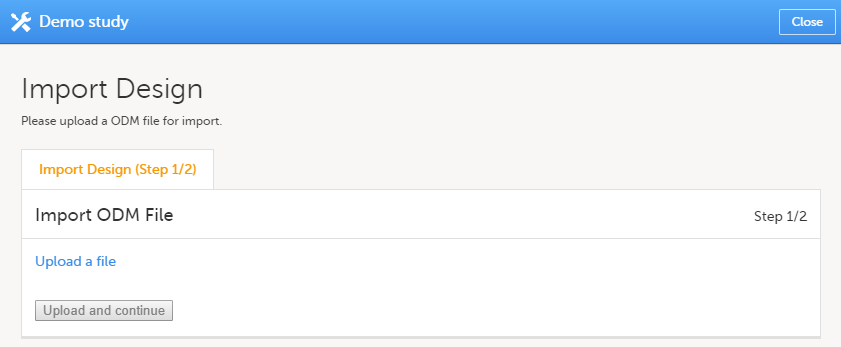
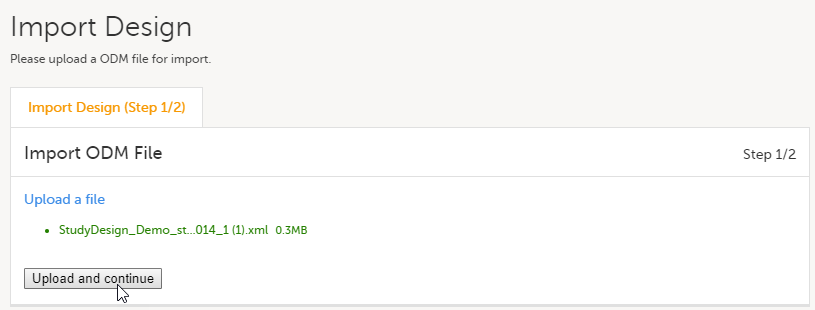
| 3 | Click the Upload a file link and select the file to be imported. |
| 4 |
Click Upload and continue: |
| 5 |
Select a design version to import - if there are more design versions in the uploaded file, choose here which one to import. Select language to import - if there are more languages available in the uploaded file, the main design language (usually English) should be chosen. |
| 6 | Click Import. You will be directed to the design overview page. For further details, see Overview of study design. |