Partial Submit Setup
This lesson describes how to configure partial submits of forms in Viedoc Designer.
Introduction
Partial submit setup
From Viedoc release 4.74, for Japanese PMS studies, Partial Submit Setup is available, which allows the study designer to configure which forms will be able to be submitted individually. Any form in use from the current design can be selected.
| Important! By default, Partial Submit Setup is disabled. If it is not enabled, the default behavior is that only AE forms (with FormID = AE) can be submitted individually. |
Setting up the partial submit is done in Viedoc Designer, under Study Settings > Partial Submit Setup.
Note! The Partial Submit Setup settings can only be edited in a new design version (not a revision).
Enabling partial submits
For clinic users to be able to partially submit other individual forms, (as opposed to AE forms only) in a booklet, you must enable Partial Submit Setup in Study Settings in Viedoc Designer.
Note!
- Enabling the Partial Submit Setup is not possible on either a revised, published, or locked version of the study design.
- When you have enabled the Partial Submit Setup in a new design version, you can edit the Partial Submit Setup page and add one or more definitions for a partial submit.
| Important! When Partial Submit Setup is enabled, only the forms with an added Partial Submit Definition can be partially submitted in Viedoc Clinic. This includes AE forms. You can choose any form in the current design that is in use, that is, it was added to the study workflow, to add a Partial Submit Definition to. |
To enable the Partial Submit Setup:
| 1 |
Open the study in Viedoc Designer. The Overview of the study design page opens. 
|
| 2 |
Select Edit in the Study Settings field to open the Study Settings page. 
|
| 3 |
The first time the Study Settings page opens, Partial Submit Setup is flagged as NOT IN USE. 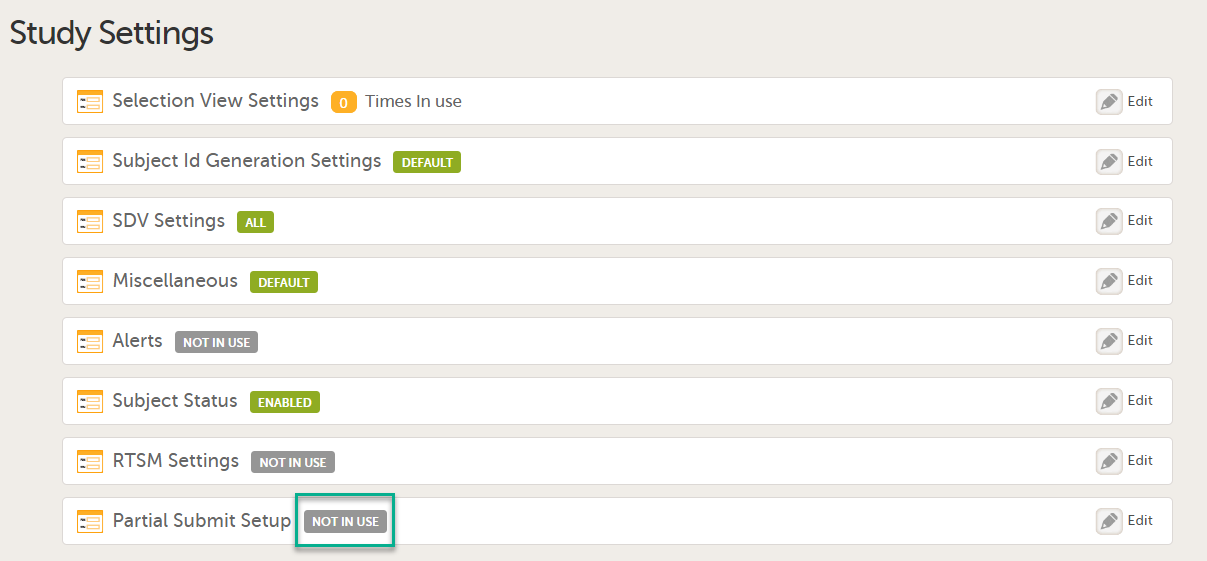
In Study Settings, select Edit in the Partial Submit Setup field. 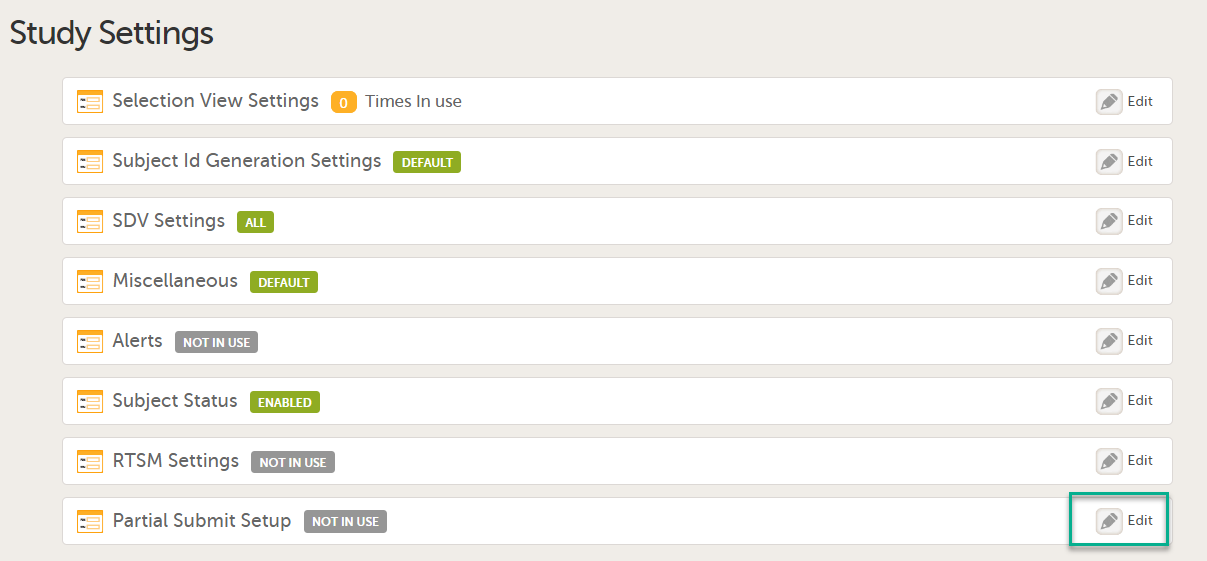
The Partial Submit Setup page opens. |
| 4 |
On the Partial Submit Setup page, select Enable Partial Submit Setup. 
|
| 5 |
A confirmation dialog appears as shown below. 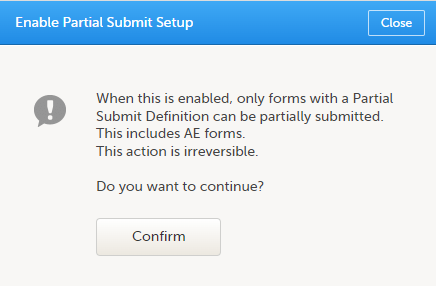
Select Confirm to continue. The Partial Submit Setup page is now available in Study Settings. Here you can add and edit one or more Partial Submit Definitions. |
Adding a partial submit definition
When you have enabled the Partial Submit Setup, follow the steps below to add a Partial Submit Definition:
| 1 |
In Study Settings, select Edit in the Partial Submit Setup field. 
The Partial Submit Setup page opens. This page lists all the existing defined partial submits for the study design. 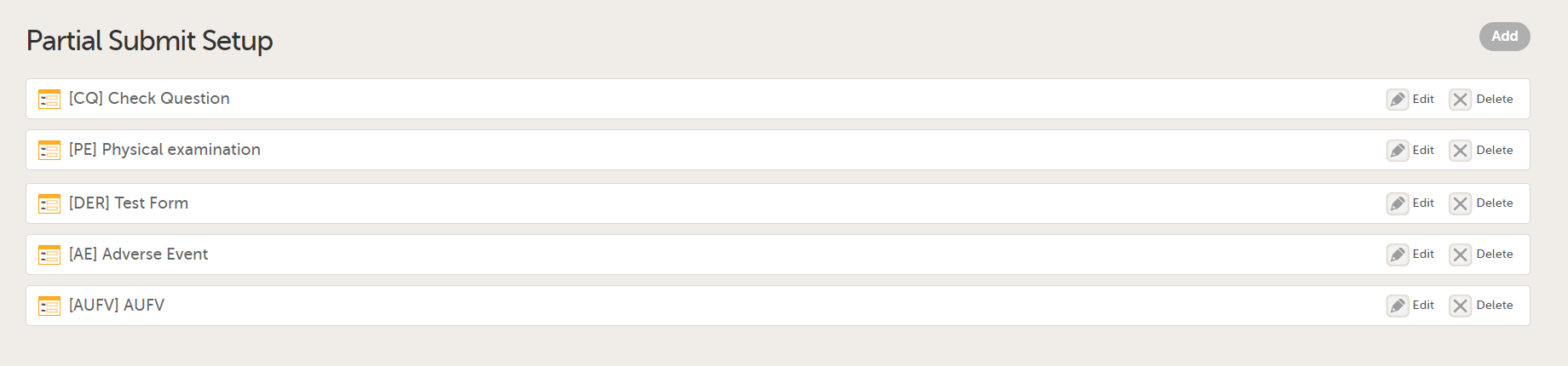
|
||||
| 2 |
Select Add to add a new Partial Submit Definition. 
|
||||
| 3 |
The Add a Partial Submit Definition page opens. Under Forms, in the Choose a form dropdown, select the form you want to add the partial submit definition to. 
Forms are labelled in the format [Form ID] Form name. You can select one or more forms to add definitions for a partial submit. You can choose any form from the list of forms in use in the current study design. Note! It is only possible to define one Partial Submit Definition per form. |
||||
| 4 |
On the Add a Partial Submit Definition page, configure the following settings as required: 1. Select as Adverse Event: Select this option to define a form as an Adverse Event. 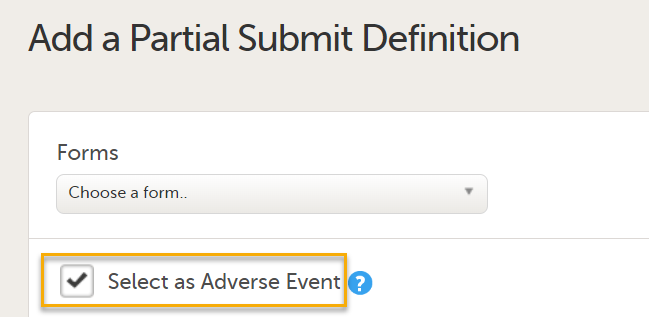
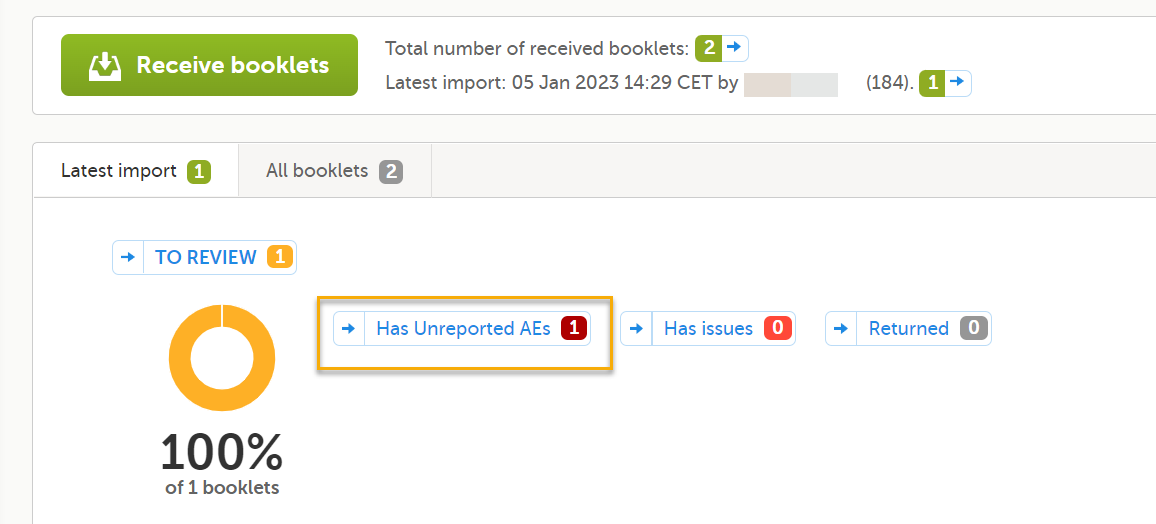
Note! Forms with Partial Submit Definitions that are selected as AEs are included in the counters for Has unreported AEs on the sponsor side shown on the Booklet overview page. See Booklet overview for more information. Unreported AE forms for studies with Partial Submit Setup enabled are counted when the following conditions apply:
2. Condition: In the Condition field, you can enter an optional JavaScript condition that must be TRUE for clinic users to be able to submit the form individually (to partially submit the form). To add a JavaScript condition: Add the condition in the Condition field: If the Condition field is not filled in, the selected form can still be partially submitted. The condition is evaluated as follows:
For more information, see Using JavaScript in Viedoc 3. Notification messages for unreported forms: There is a mandatory notification message for both the clinic and the sponsor side users with the following default text: 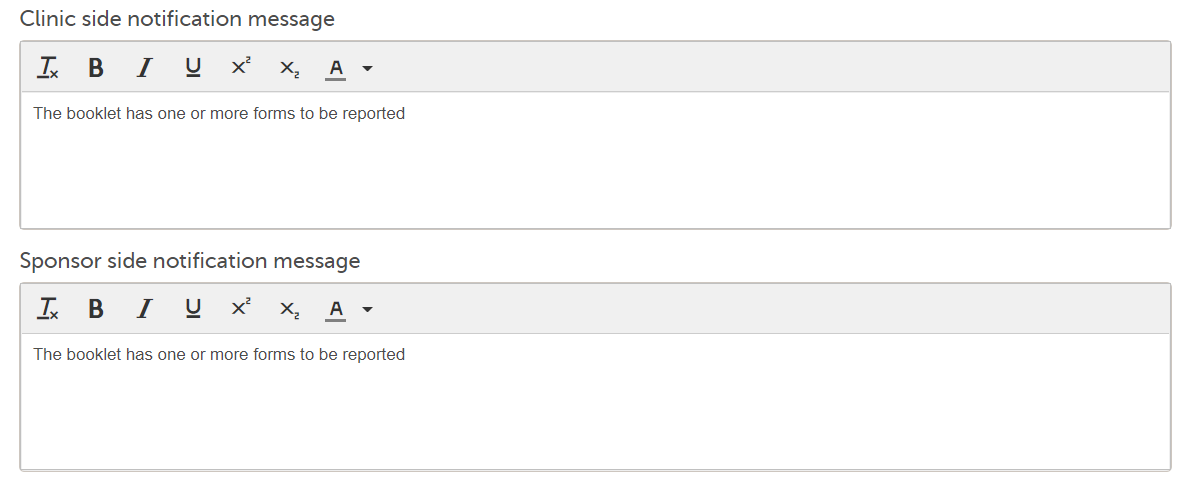
Note! Although the default message text shown in Viedoc Designer is displayed as black, the default message text in clinic is red: 
You can add a customized notification message to be shown on the clinic side or on the sponsor side for each Partial Submit Definiton. To edit the notification message, enter your text in either the clinic side or sponsor side notification message field. You can also customize the font size, color, and style of the text: 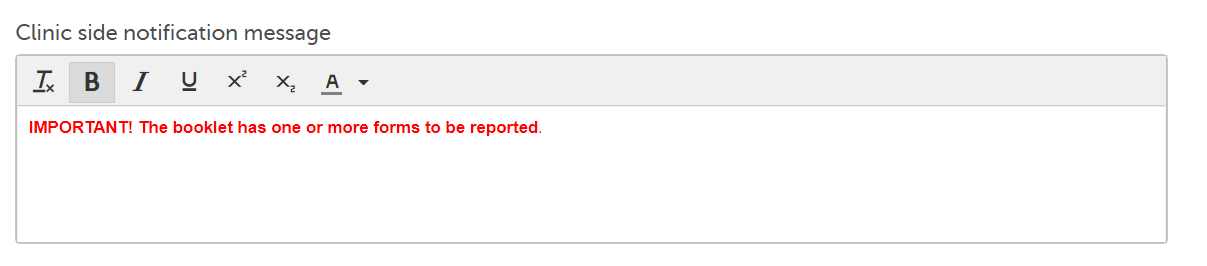
Note! The messages are included in the Complete Configuration report only. |
||||
| 5 |
Select Save changes. You will be directed to the Partial Submit Setup page where a confirmation message is displayed: 
|
Changes to Partial Submit Setup in a new design version
Partial Submit Setup can be configured only in a new design version, that is, it is not possible in a revised version.
Partial Submit Setup is always read from the current effective design applied to a site. For more information about the "current effective design" see, Settings read from current effective design.
For existing studies in Viedoc Clinic, the following applies to updates to forms in a new study design.
When a new study design version is assigned, with a form that is configured to be submitted individually, all of the existing (saved) forms can be submitted individually and the warning message is shown.
When a new study design version is assigned, where it is no longer possible to individually submit a form, the following applies for the existing (saved) forms:
- Clinic users can still view the previously saved forms but cannot submit forms.
- There are no warning messages displayed on the subject details page for that form.
- The Not submitted message on the subject details page is not shown.
- The Manage link can still be selected to show the complete history of the form's submit-receive-return actions, if the form was individually submitted, but not if the form was part of a booklet submission.
Editing a partial submit definition
You can edit a Partial Submit Definition when the Study Settings can be edited, that is, in a new design version which is unpublished and unlocked and it is not a design revision. The Partial Submit Definition can still be viewed if the study design is a revision, published or locked.
Note! On the Study Settings page, for the Partial Submit Setup, a counter shows the number of existing partial submit definitions. Each existing partial submit definition is labelled as:
- NOT IN USE - if a Partial Submit Definition has not been enabled in the study settings.
- In use - if a Partial Submit Definition has been enabled in the study settings.
- If enabled, X Times In use is shown, where X is the number of defined partial submits.
| 1 |
To edit an existing form with a partial submit definition, select Edit on the form you want to edit on the Partial Submit Setup page. The Edit Partial Submit Definition page opens. 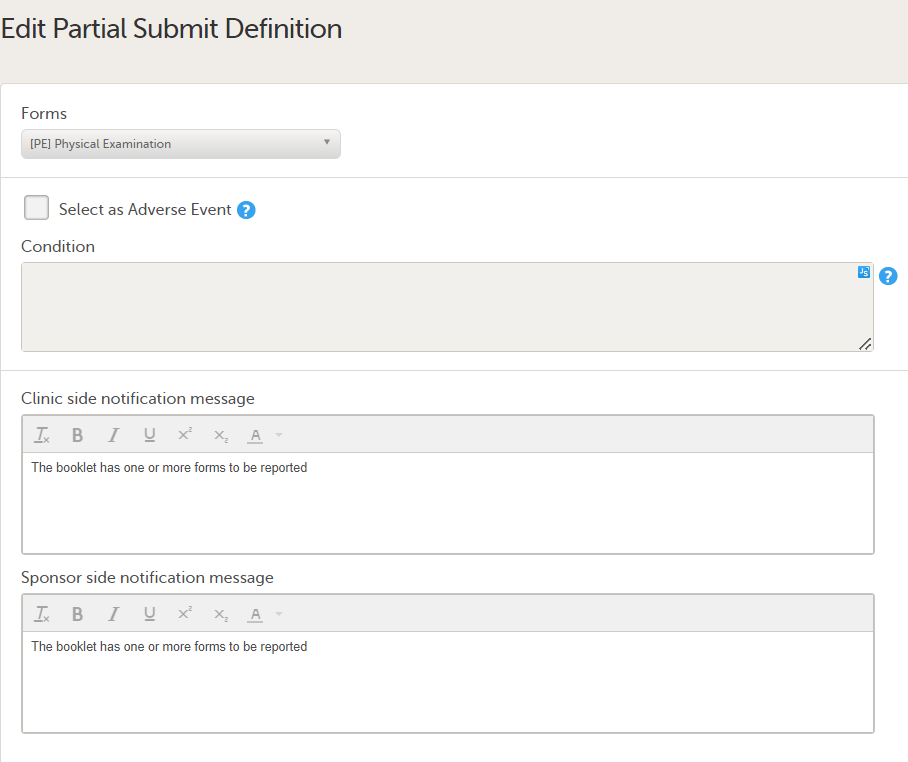
|
| 2 |
In the Forms dropdown menu, select the form you want to edit. You can choose any form from the list of forms in use in the current design: 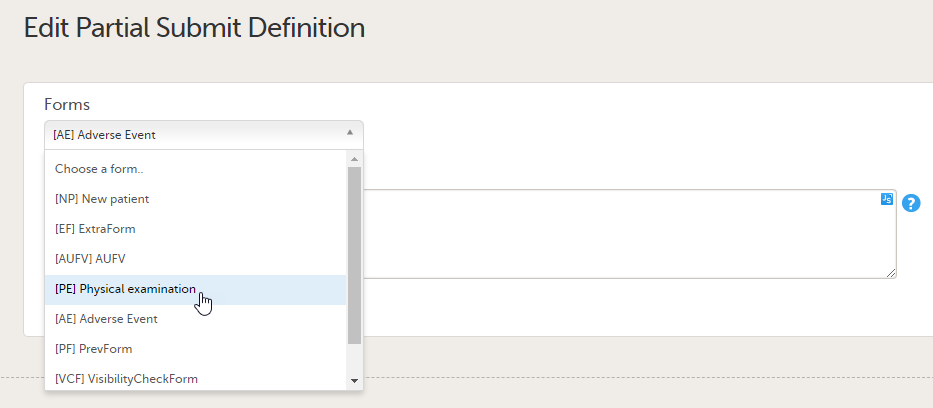
Note! The form name is not visible if the form ID has been changed, or the form has been deleted from the study design. If a form name or the form ID has been changed, an error message is displayed when validating the study design: 
|
| 3 |
When editing the Partial Submit Definition, the default notification message for the clinic side and the sponsor side is shown as described above. To edit the notification message, enter your text in either the clinic side or the sponsor side notification message field: 
|
| 4 |
Select Save changes. You will be directed to the Partial Submit Setup page where a confirmation message is displayed: 
|
Deleting a partial submit definition
You can delete a Partial Submit Definition from Study Settings>Partial Submit Setup when the study design can be edited, that is, it is not a design revision and is unpublished and unlocked.
Note! Before deleting a Partial Submit Definition please read the following information:
- If an existing partial submit definition is deleted, the existing forms with partial submissions in Viedoc Clinic are visible to clinic/site users, and the Manage link remains available to open the Manage Form window and show the History. No kaifu actions are possible and no messages are shown on the clinic or the sponsor side.
To delete a Partial Submit Definition, follow the steps below:
| 1 |
Select Delete on the form with the partial submit definition you want to remove. 
|
| 2 |
A message is shown asking for confirmation: 
Select Yes to delete the form. After deletion, the original selected form for the deleted partial submit definition will still be available if you want to add a new partial submit definition to that form. Note! The Delete button is disabled when the study settings are in view mode, which is when the study design cannot be edited. |
Configuration report
The Partial Submit Setup configuration is available in the Excel configuration report of the study design, to give a complete overview of the study design version. In Viedoc Designer, the complete configuration report contains all the settings configured in Study Settings.
For more information, see Configuration report.