Configuration report
Introduction
You can download a report of the study design in an abbreviated or complete version.
- The abbreviated version is a short summary in PDF format.
- The complete version is a detailed Excel file.
To download one of the files, select Abbreviated or Complete on the study design overview page:
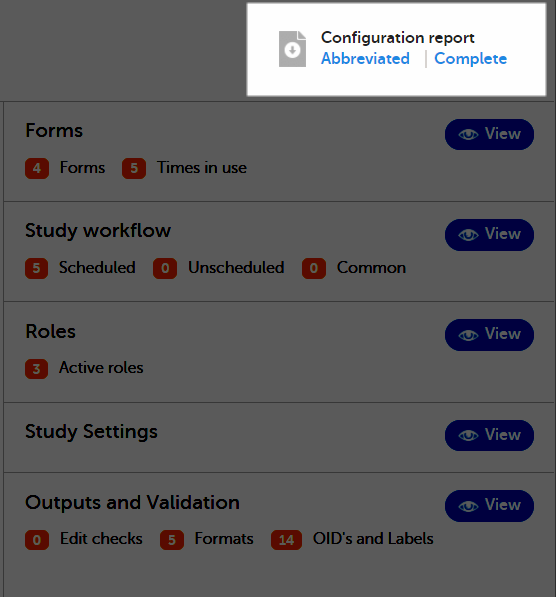
Abbreviated PDF report
The configuration report in PDF format contains a summary of the following settings within the study design:
- Design details
- Languages
- Forms
- Study workflow
- Roles
- Study settings
- Edit checks
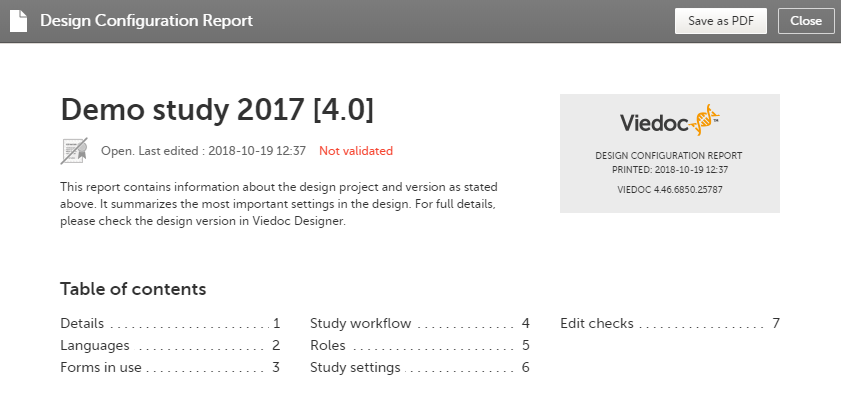
For Japanese PMS studies, the Abbreviated Configuration report also shows whether the Partial Submit Setup is enabled, and lists the existing partial submit definitions:
Complete Excel report
The configuration report in Excel format is a detailed report of the study design. Each sheet corresponds to the settings made in Designer, as shown in the following table:
| Sheet | Corresponding section in Designer |
|---|---|
| Design info |  |
| Design settings |  |
| Global-Designer settings |  |
| Global-Medical coding | |
| Global-Data mappings | |
| Global-Reference data scopes | |
| Global-Reports configuration | |
| Settings-Selection view |  |
| Settings-Subject Id Generation | |
| Settings-SDV | |
| Settings-Miscellaneous | |
| Settings-Alerts | |
| Settings-Subject Status | |
| Settings-RTSM | |
| Settings-eLearning | |
| Settings-Partial Submit Setup (Japanese PMS studies only) | |
| Roles |  |
| Forms | 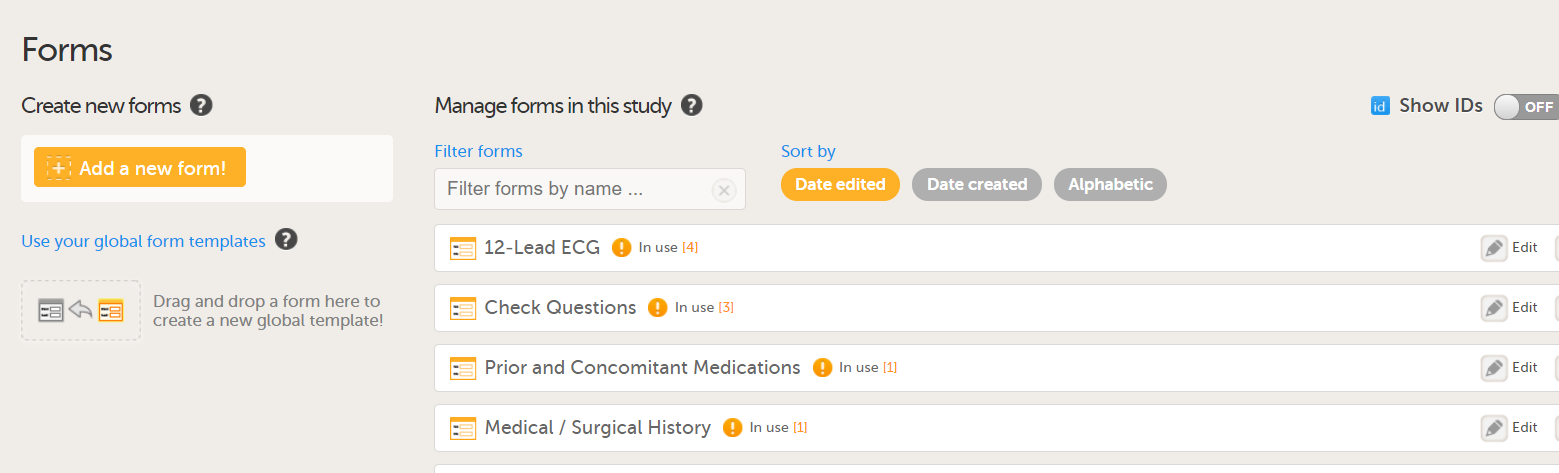 |
| Items and Groups |  |
| Study workflow-Events | 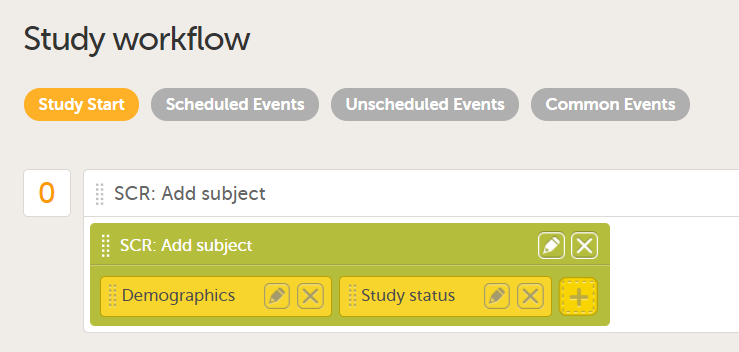 |
| Study workflow-Activities | |
| Study workflow-Forms | |
| Viedoc Me reminder | 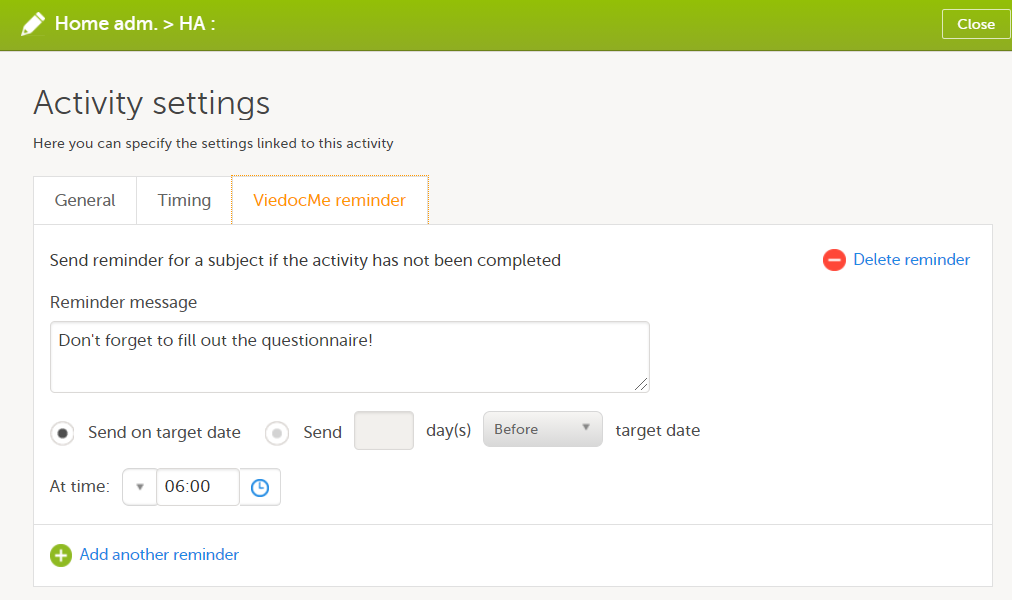 |
| Functions and Conditions |
|
| Data checks | 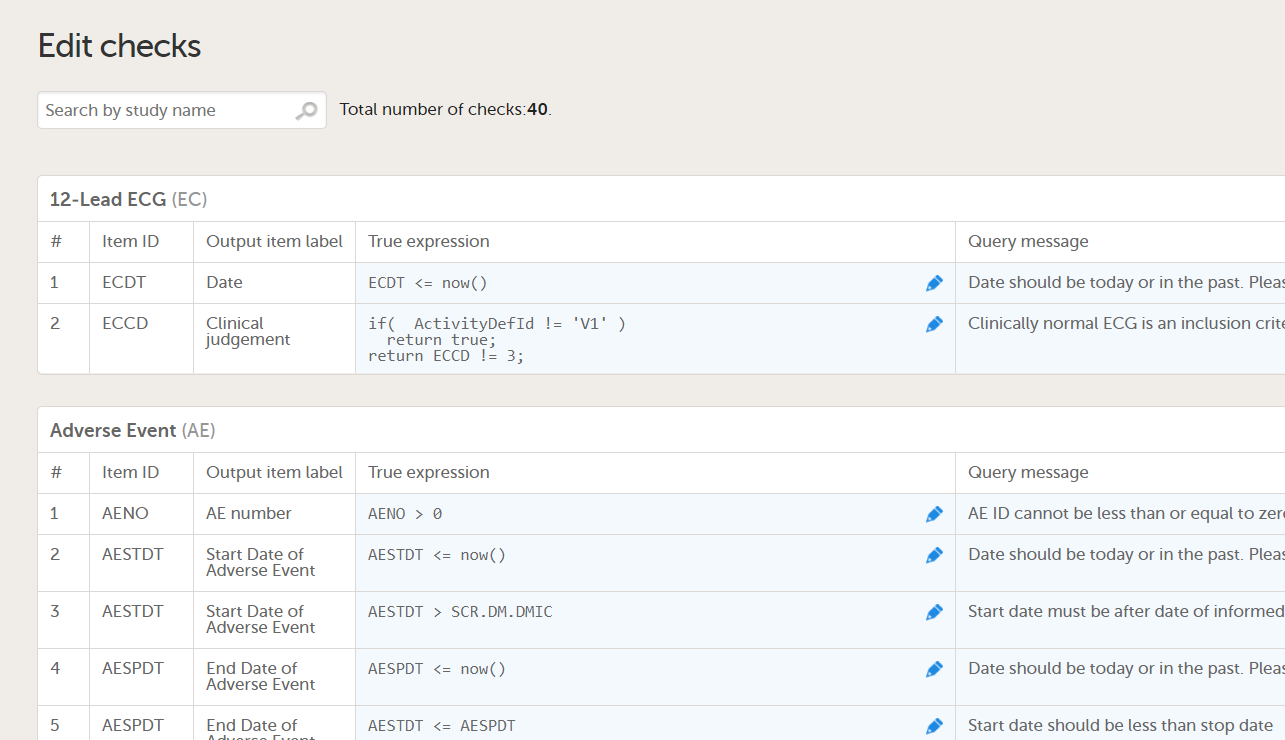 |
| Code lists |  |
The report is self-explanatory but in the following sections you can find useful tips on how to navigate and understand some content of the file:
- Global-Data mappings
- Settings-SDV
- Settings-Alerts
- Settings-RTSM
- Settings-Partial Submit Setup
(for Japanese PMS studies only) - Forms
- Items and Groups
- Study workflow-Events
- Study workflow-Activities
- Study workflow-Forms
- Viedoc Me reminder
- Data checks
Global-Data mappings
In Designer, if data mappings are defined:
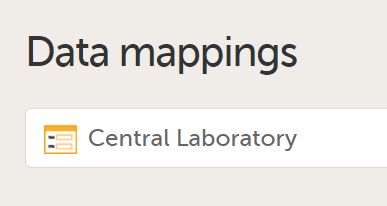
...they show up in the Global-Data mappings sheet:
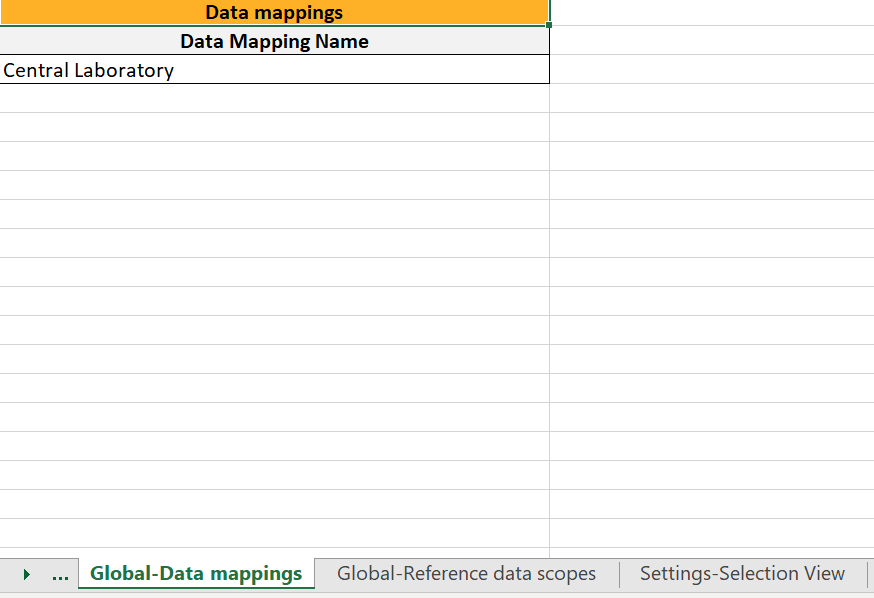
Note! Only the names of the data mappings are listed. For details about a data mapping, refer to the Define-XML file.
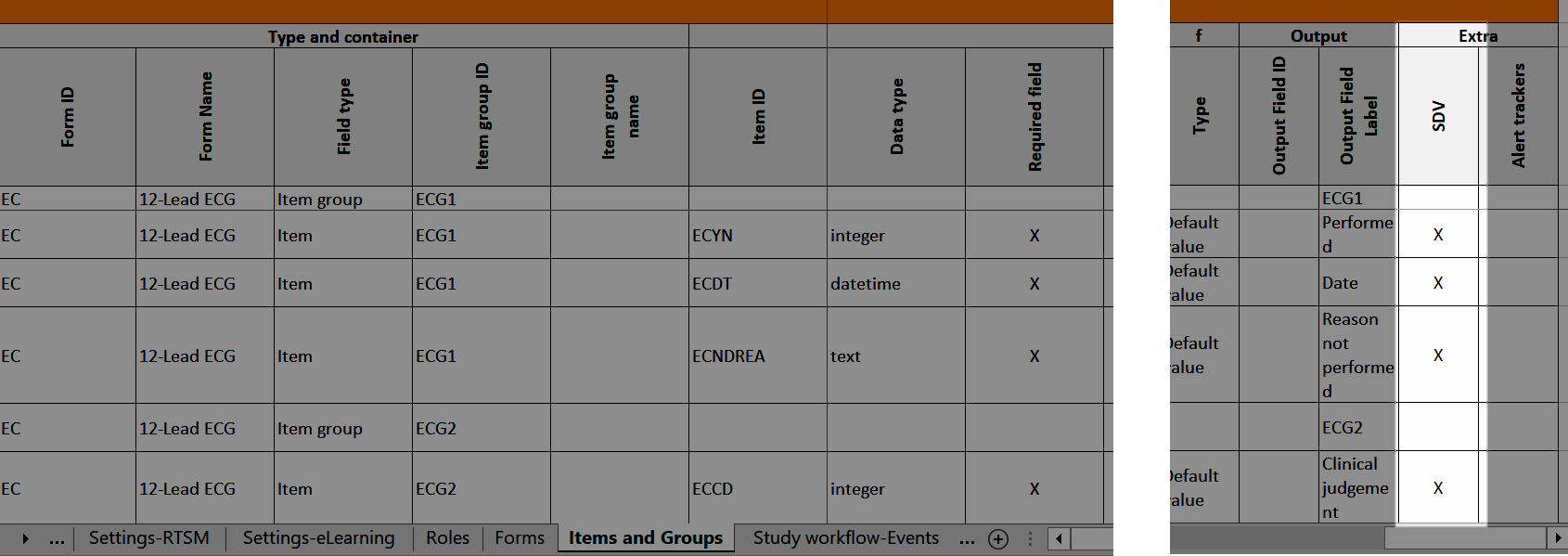
Settings-SDV
In Designer, if Include single forms and items is selected in the SDV Settings:
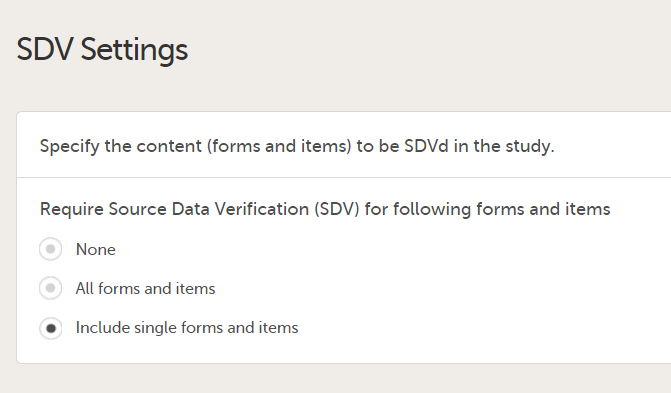
...it is marked (X) in the SDV Settings sheet:
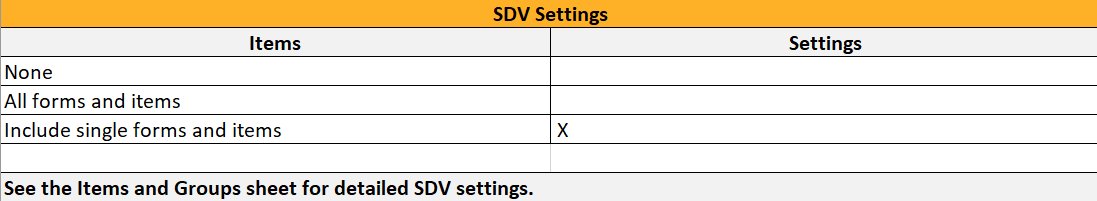
...and items with SDV settings are marked (X) in the Items and Groups sheet in the SDV column:
Settings-Alerts
In Designer, if alerts are set in Study Settings:

...they show up in the Settings-Alert sheet:
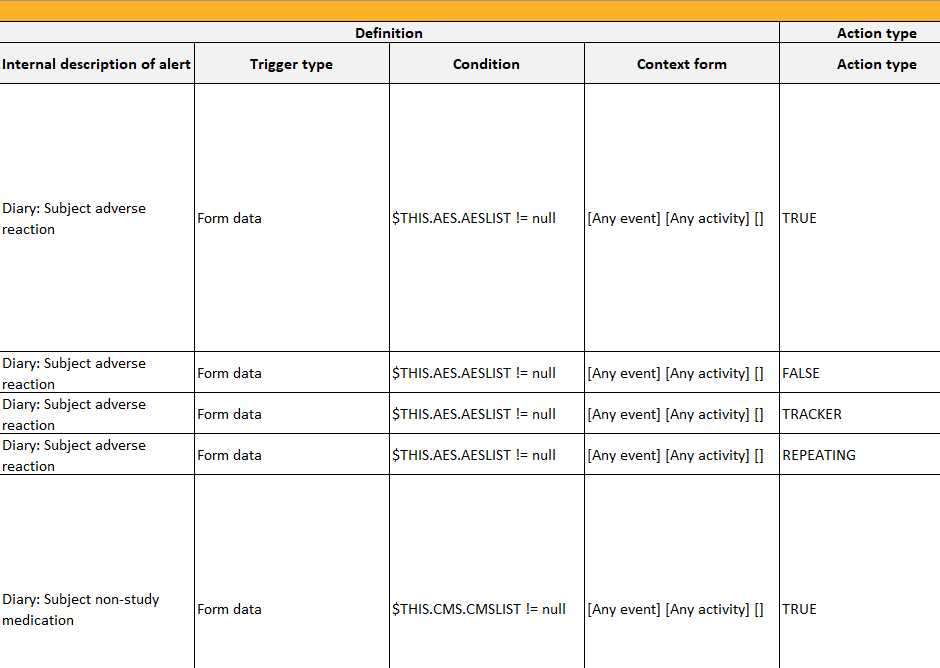
Alert trackers
In Designer, if alert trackers are set:
...they show up in the Items and Groups sheet:
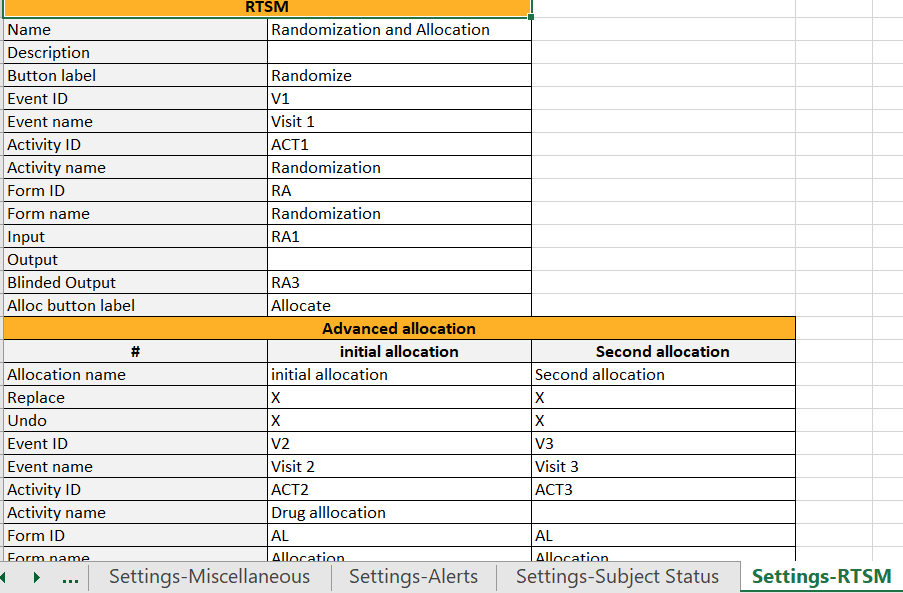
Settings-RTSM
In Designer, if RTSM settings are set:
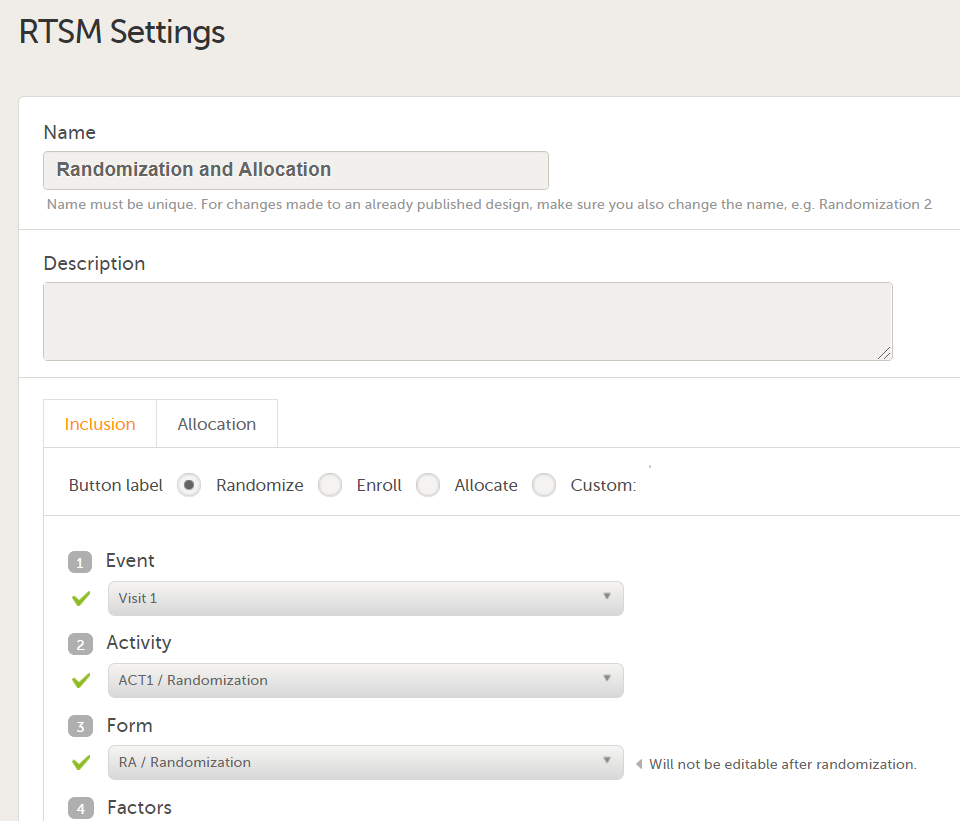
...they show up in the Settings-RTSM sheet:
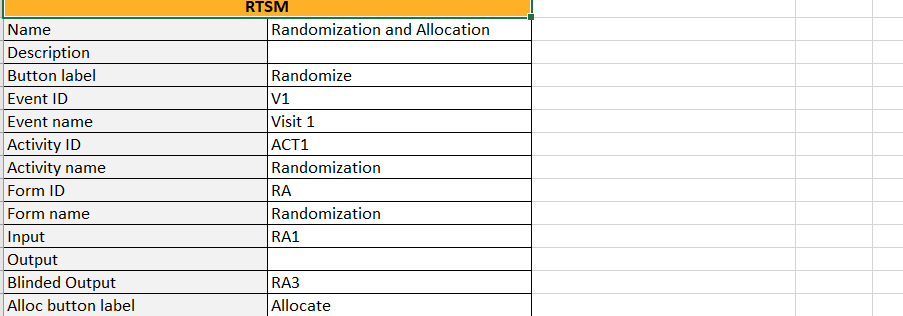
...with advanced allocation settings showing up below the RTSM settings, column-wise:
Settings Partial Submit Setup
In Designer, for Japanese PMS studies only, in the Complete Configuration report, the Partial Submit Setup:
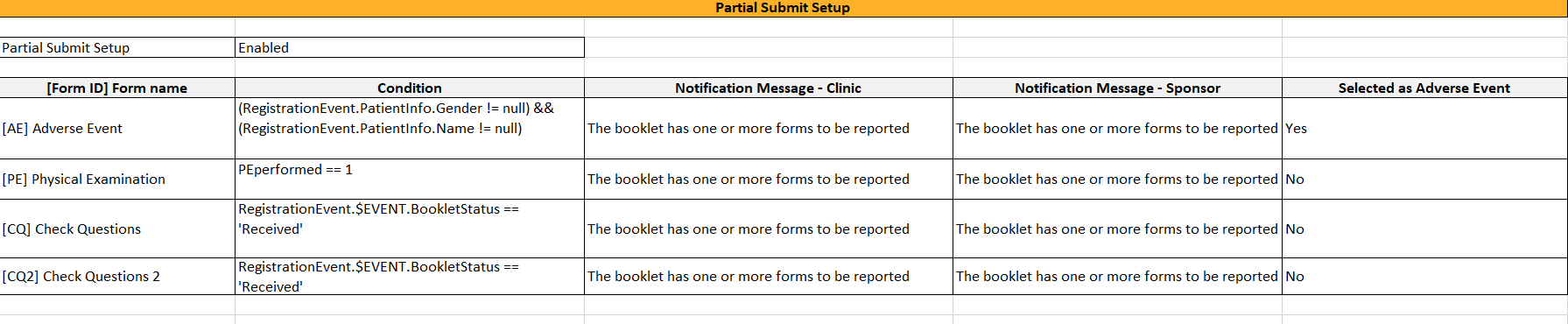
shows up in the Settings-Partial Submit Setup sheet:
The Settings-Partial Submit Setup sheet contains the following information:
- Partial Submit Setup - Enabled/Not enabled
- A list of the existing partial submit definitions; for each definition the following information is available:
- [Form ID] Form name
- Condition
- Clinic notification message
- Sponsor notification message
- Selected as Adverse Event
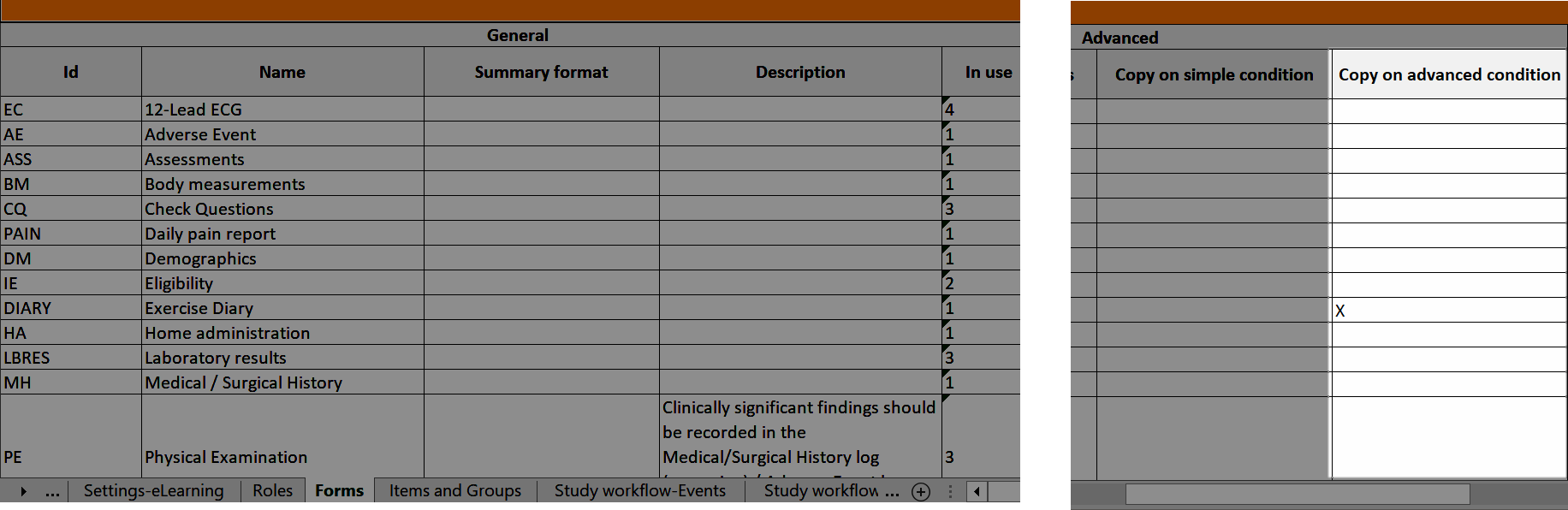
Forms
Copy on advanced condition
In Designer, if a copy on advanced condition is defined, it is marked (X) in the Copy on advanced condition column:
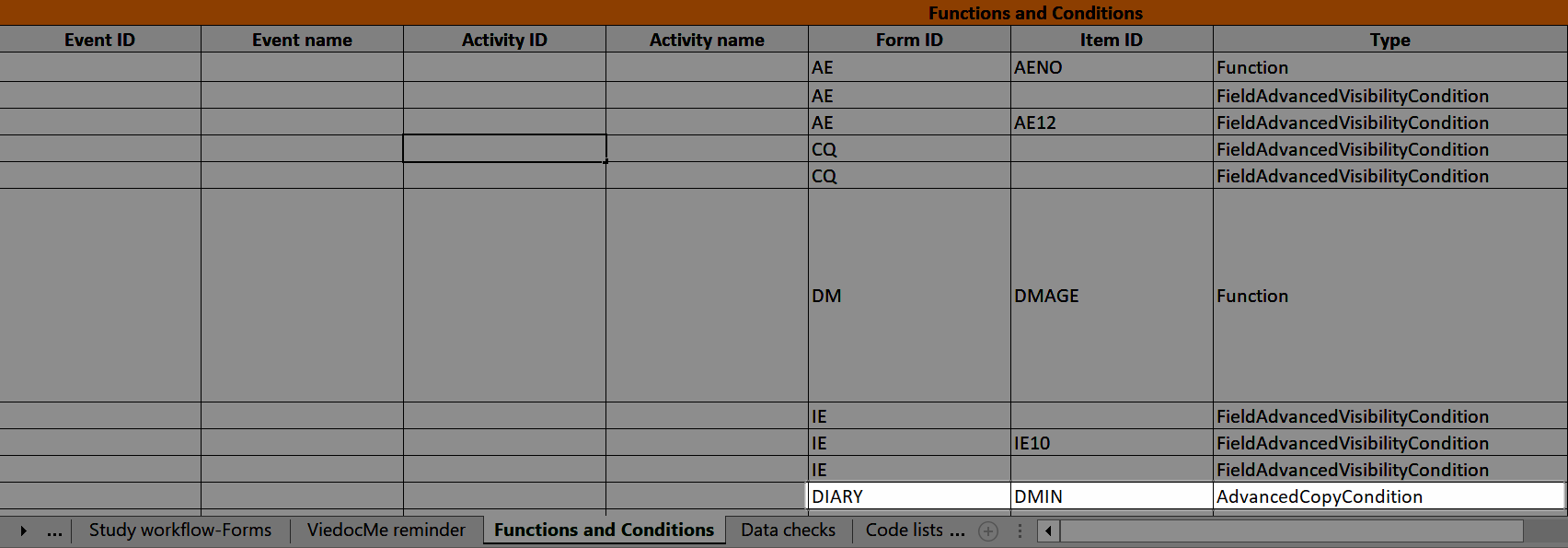
...and the definition of the copy condition shows up in the Function and Conditions sheet in the Type column as AdvancedCopyCondition:
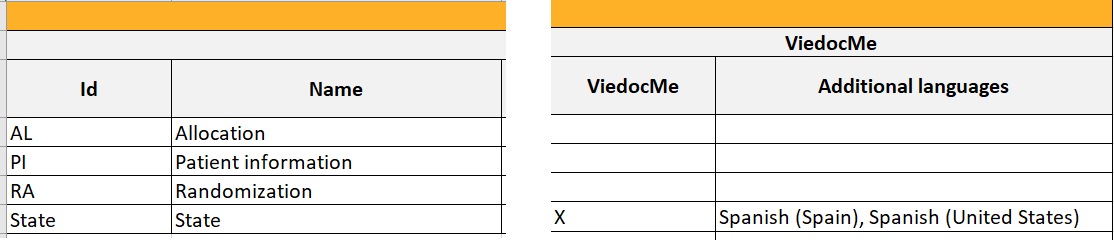
Forms included in Viedoc Me events
Forms that are included in subject-initated events are marked (X) in the Viedoc Me column. If a Viedoc Me form is translated, the languages and their cultures show up in the Additional languages column.
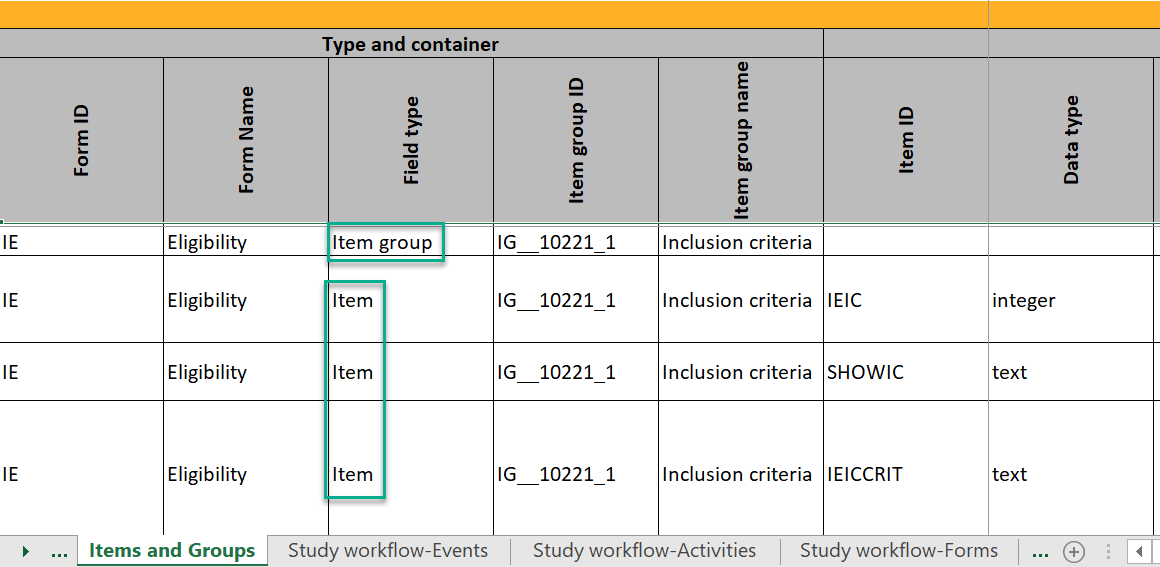
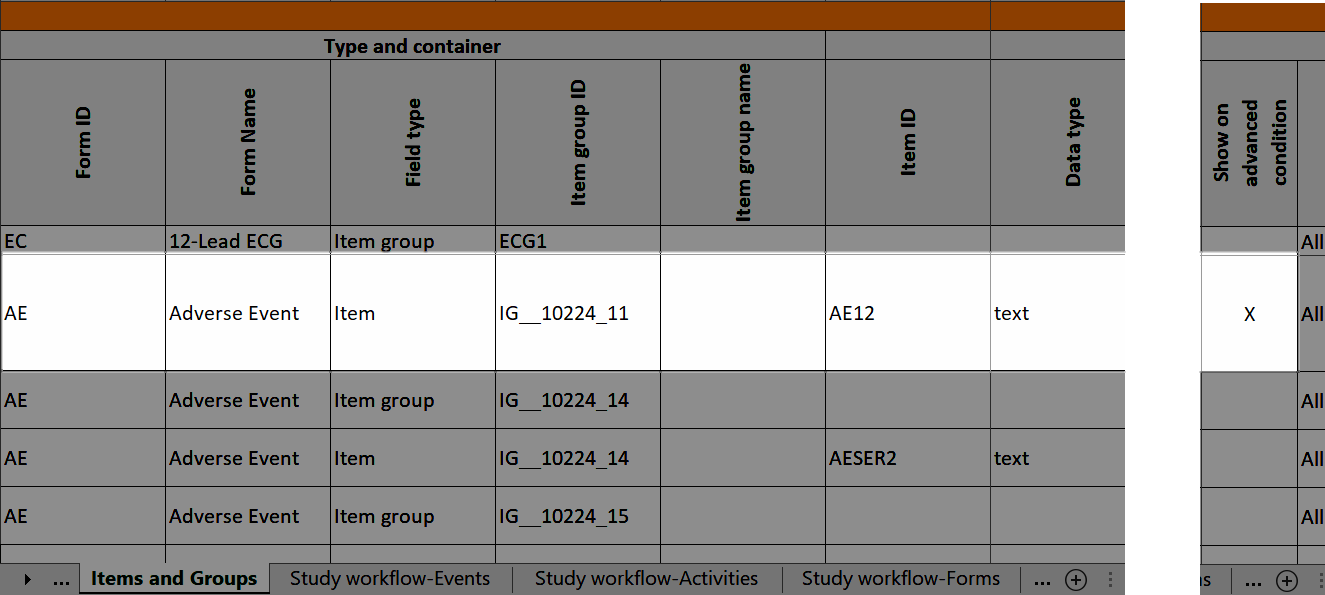
Items and Groups
The Items and Groups sheet shows all the properties that are set for the design items, thus, only some of the properties apply to an individual item.
Item group ID and Item group name identify the item groups. They also specify what item group the individual items are located in. In the below example, an item group named "Inclusion criteria" is specified. The next row specifies an item named "IEIC" that is located inside the item group:
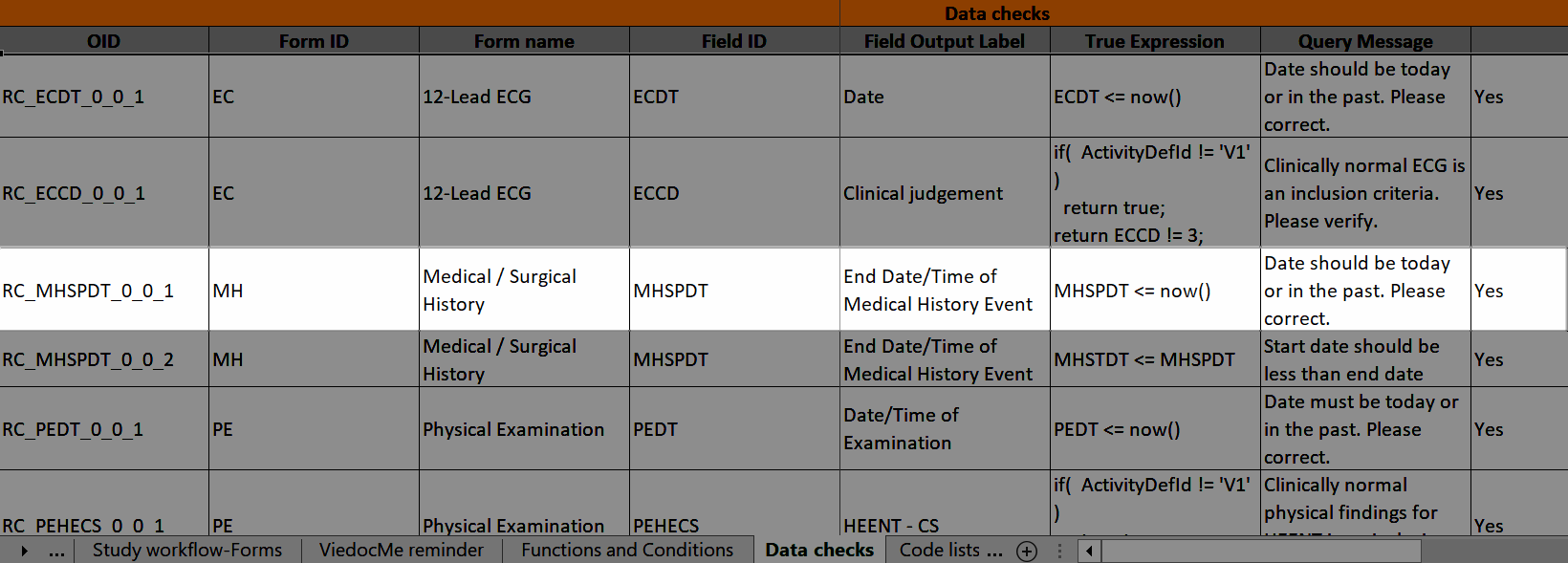
Data checks
The Data checks column shows if data checks (edit checks) are implemented:
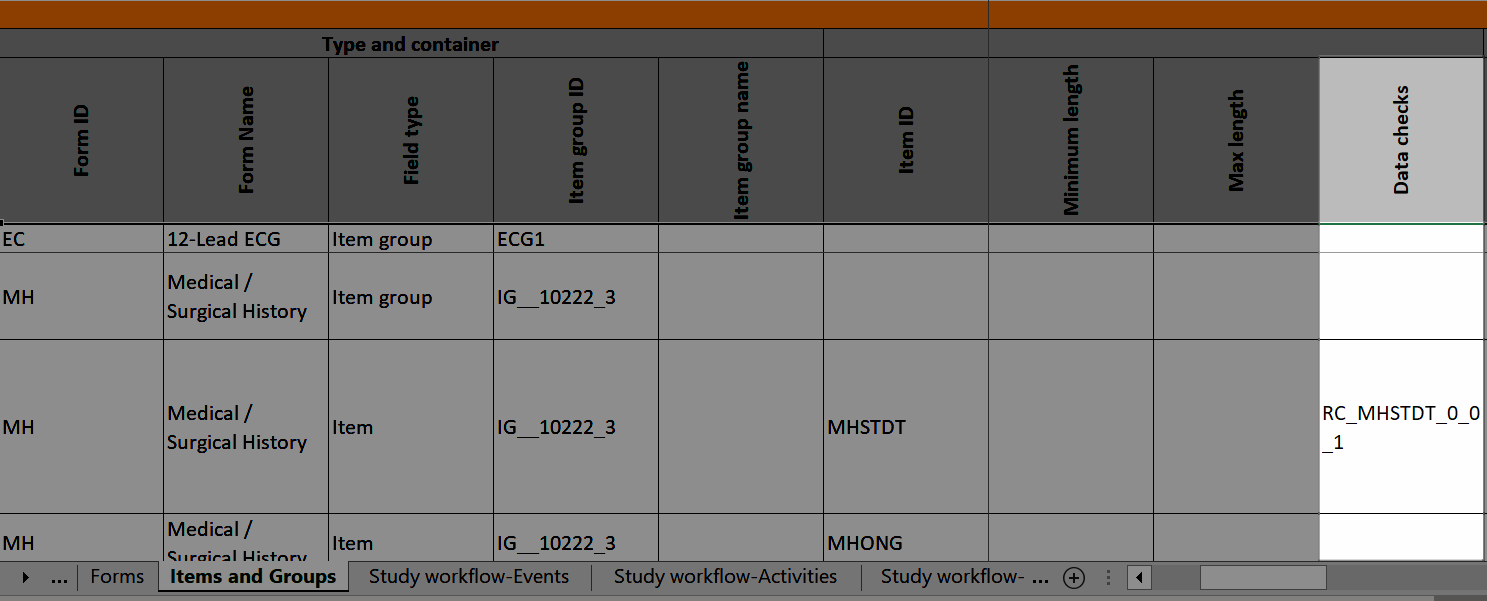
...and the definition of the data check in JavaScript shows up in the Data checks sheet:
Show on advanced condition
In Designer, if an advanced visibility condition is defined:
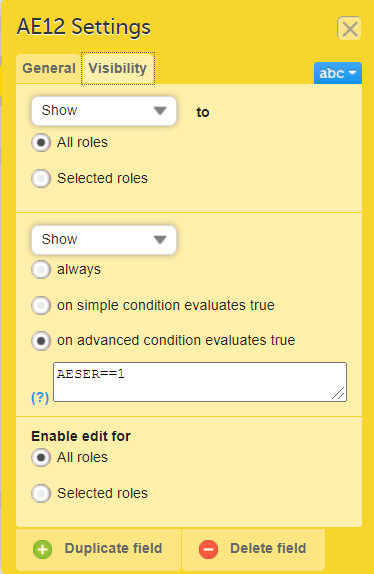
...it is marked (X) in the Show on advanced condition column:
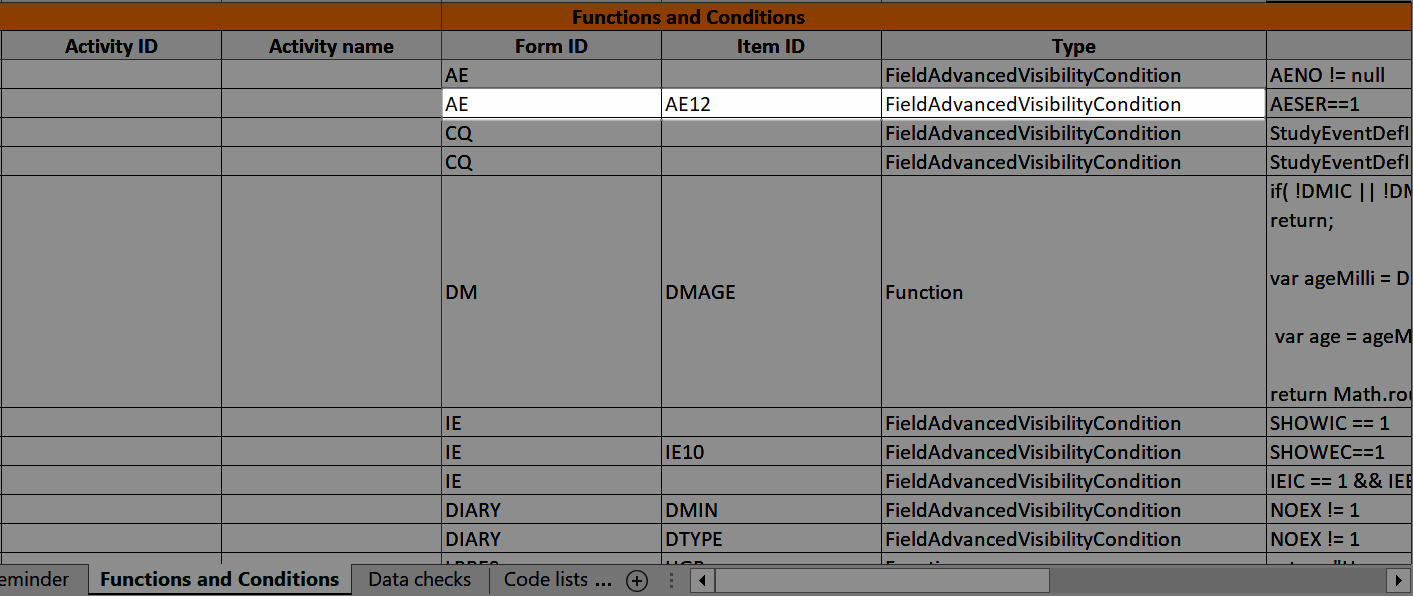
 ...and the definition of the advanced visibility condition shows up in the Functions and Conditions sheet in the Type column as FieldAdvancedVisibilityCondition:
...and the definition of the advanced visibility condition shows up in the Functions and Conditions sheet in the Type column as FieldAdvancedVisibilityCondition:
Hidden in activity
In Designer, if an item is hidden in an event/activity:
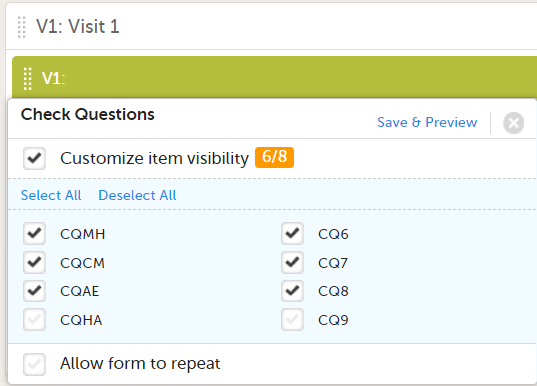
...the event/activity in which the item is hidden shows up in the Hidden in activity column:
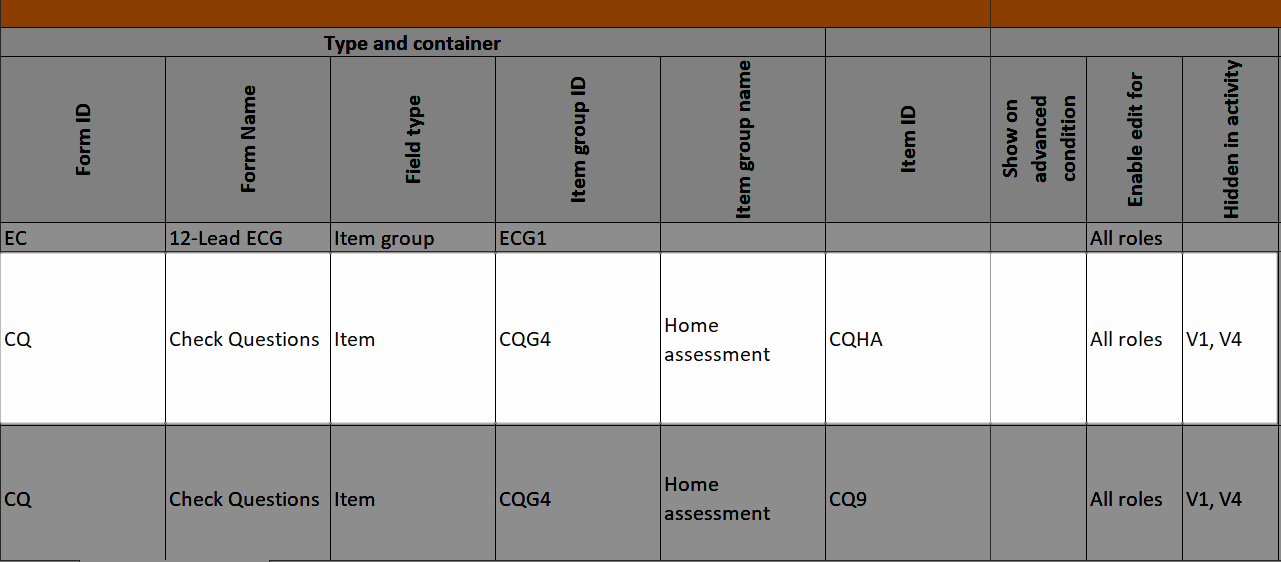
...and the hidden items show up in the Study workflow-Forms sheet in the Hidden items column:
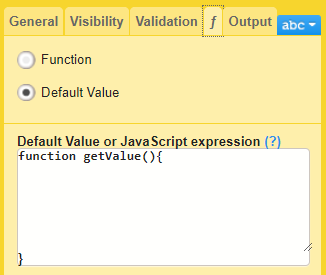
f-Type
In Designer, if a function or a default value is set to initiate an item:
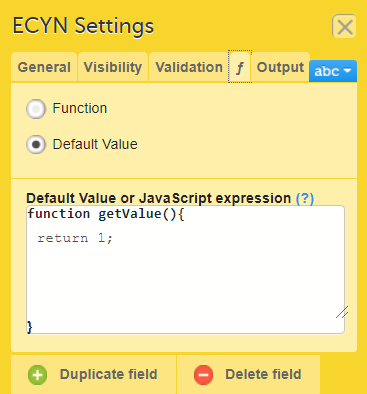
...it shows up in the f-Type column:
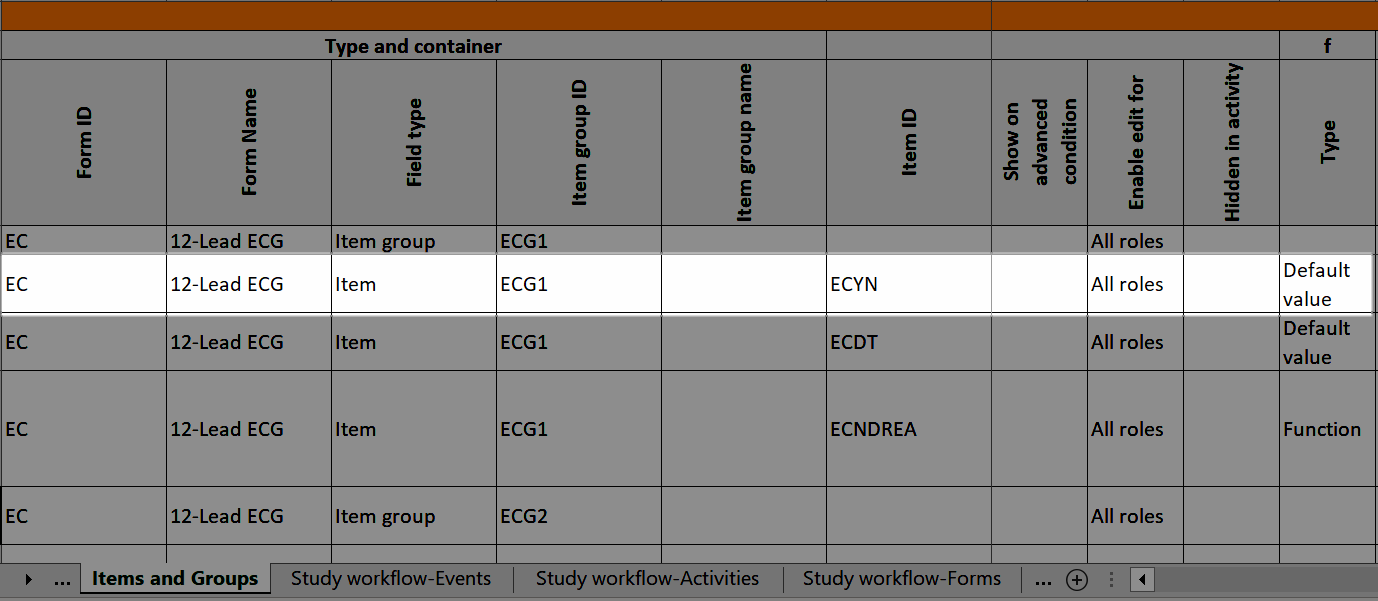
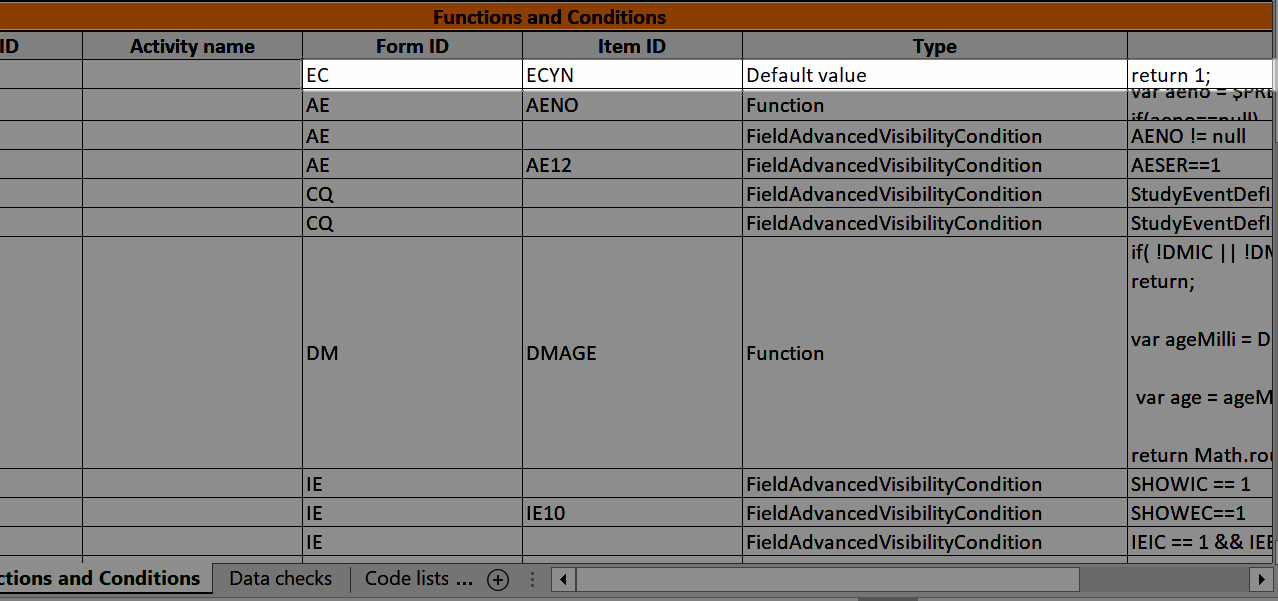
...and the definition of the default value or function shows up in the Functions and Conditions sheet:
SDV
The SDV column shows if the item is set to be SDV:d, see Settings-SDV.
Alert trackers
The Alert trackers column shows the name of the trackers that specify tracking on the item, see Alert trackers.
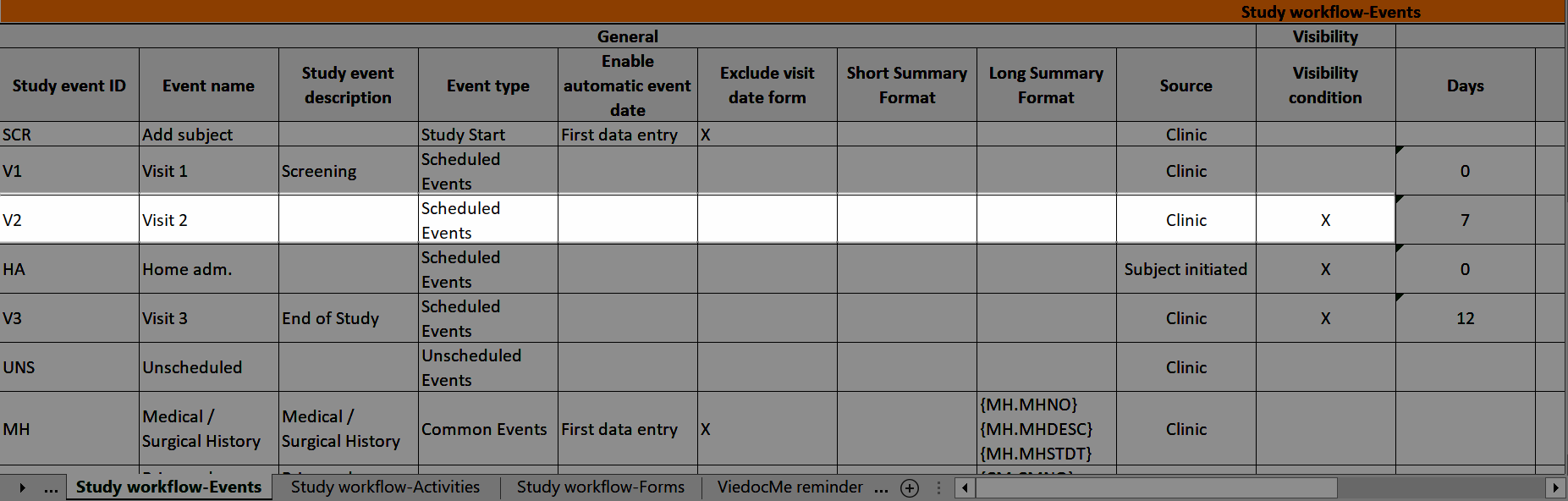
Study workflow-Events
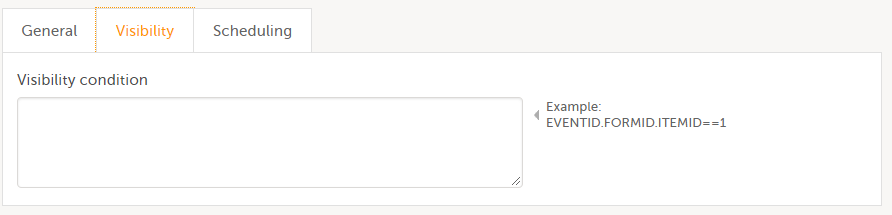
Visibility condition
In Designer, if a visibility condition is defined:
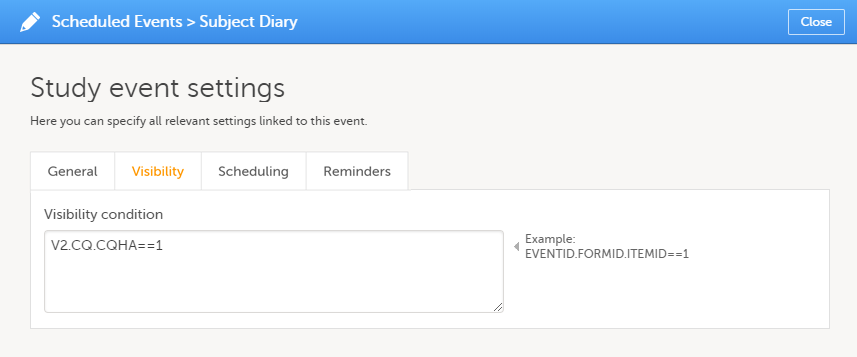
...it is marked (X) in the Visibility condition column:
...and the definition of the visibility condition shows up in the Functions and Conditions sheet in the Type column as EventAdvancedVisibilityCondition:
Study workflow-Activities
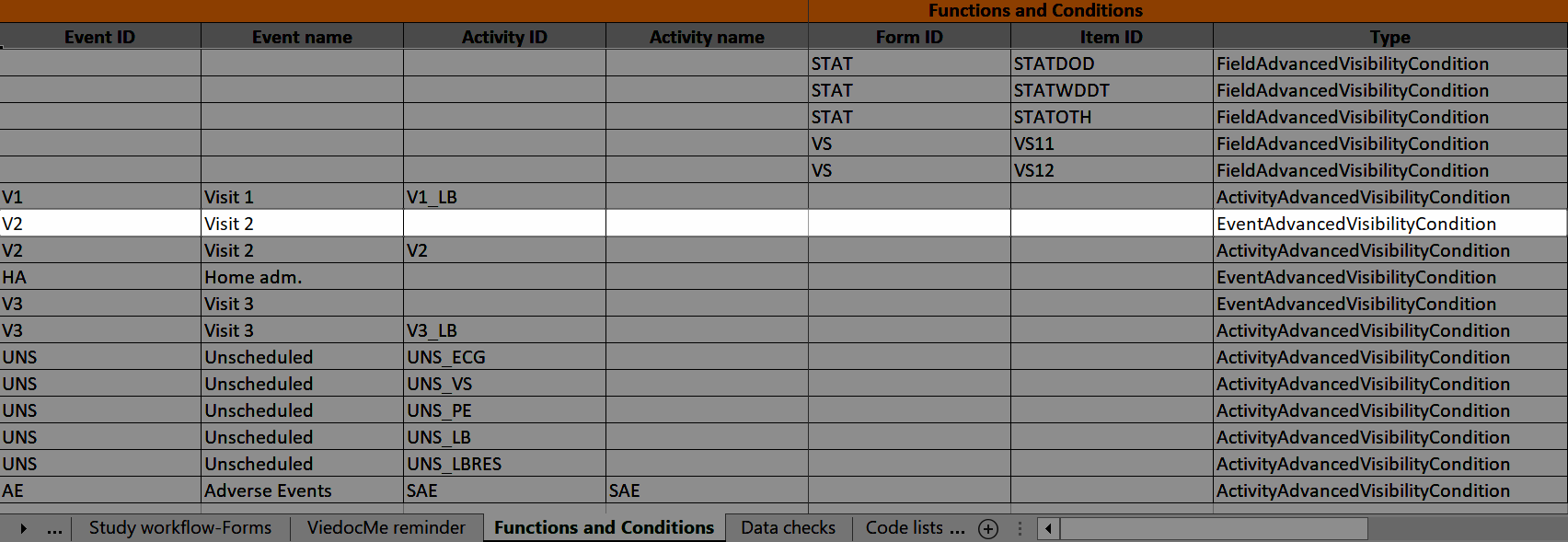
The Study event ID and Event name columns show what event the activity belongs to:
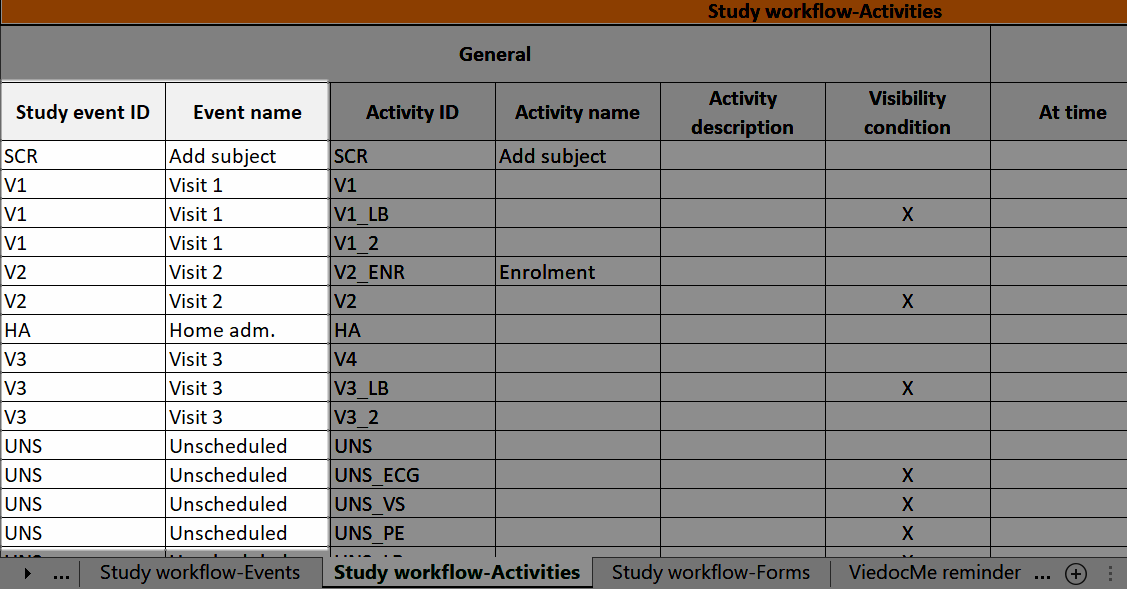
Visibility condition
In Designer, if a visibility condition is defined:
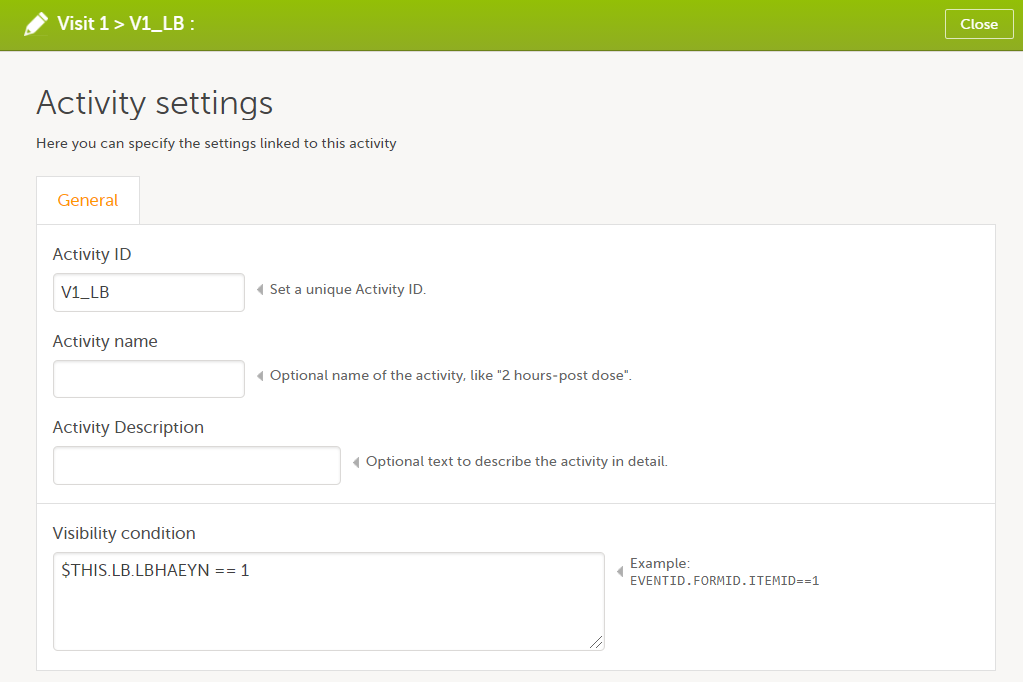
...it is marked (X) in the Visibility condition column:
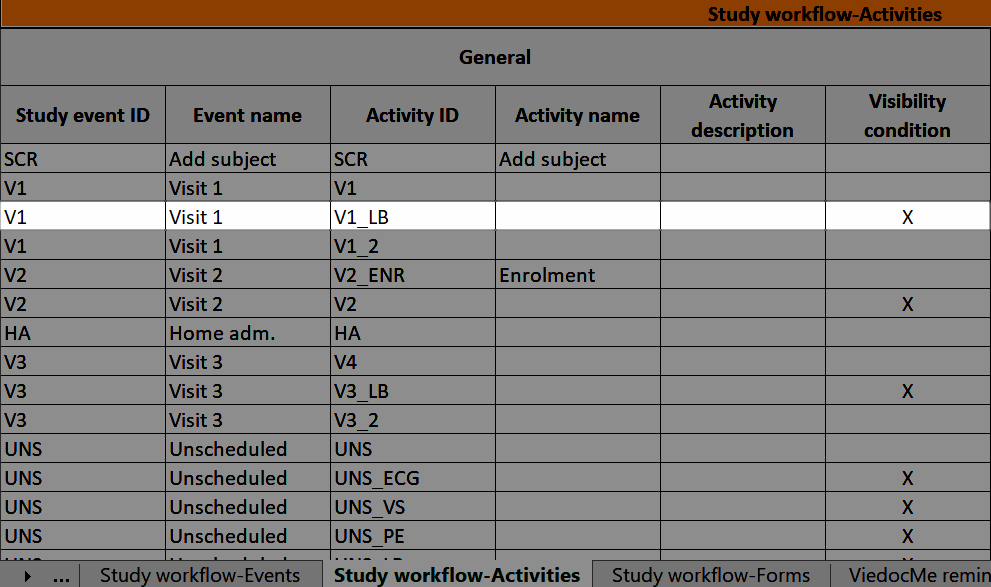
...and the definition of the visibility condition shows up in the Functions and Conditions sheet in the Type column as ActivityAdvancedVisibilityCondition:
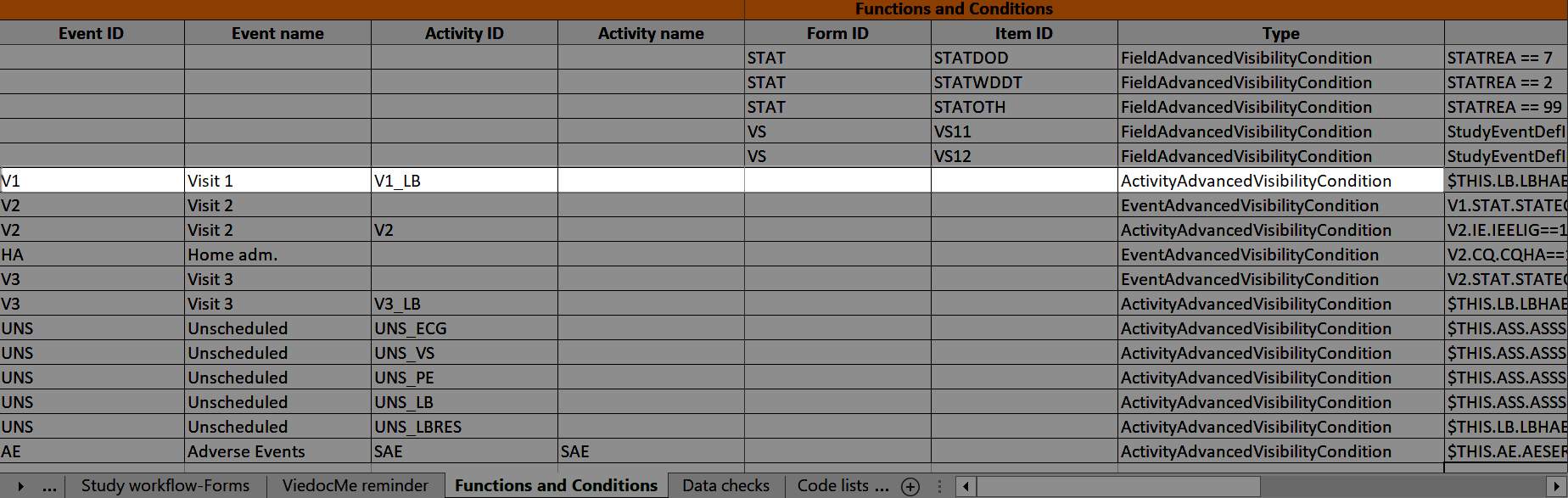
Viedoc Me reminder
The Viedoc Me reminder column shows if the activity defines a Viedoc Me reminder. The reminder settings show up under the Viedoc Me reminder sheet, see Viedoc Me reminder.
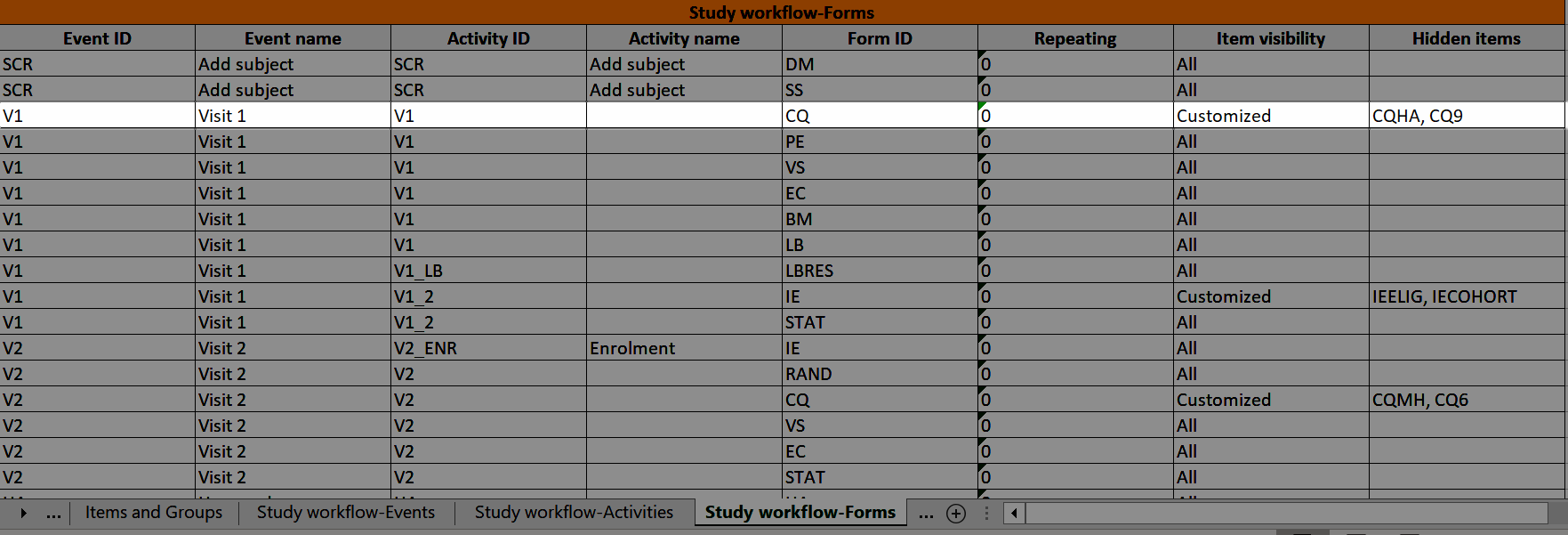
Study workflow-Forms
The Event ID, Event name, Activity ID, and Activity name columns show which event and activity the form belongs to:
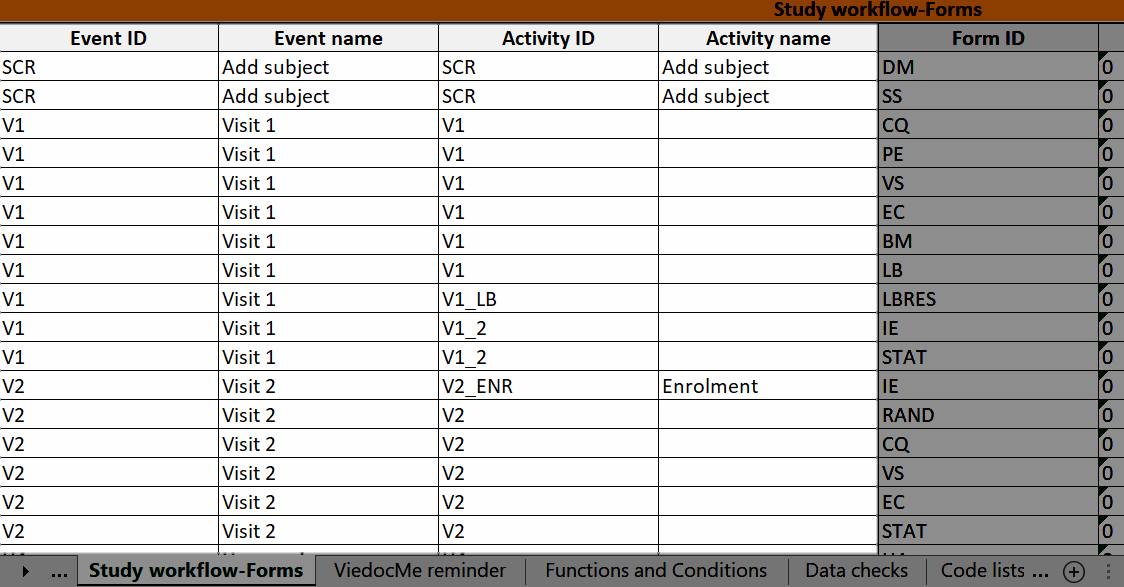
Viedoc Me reminder
In Designer, if Viedoc Me reminders are set:

...they show up in the Viedoc Me reminder sheet:
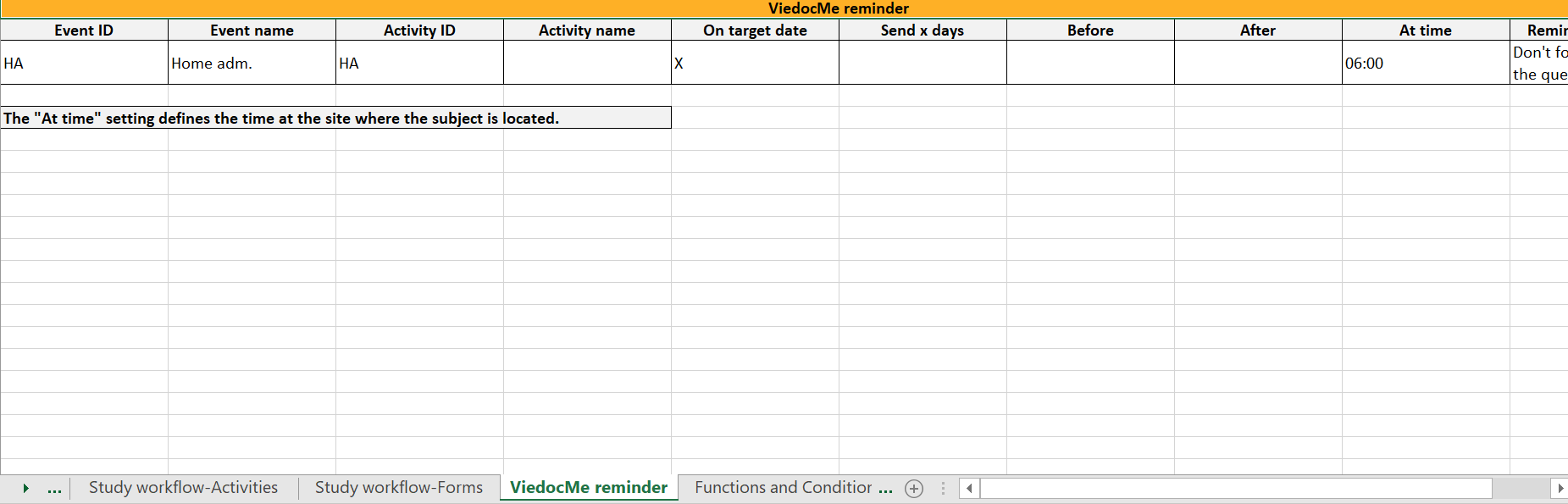
The Event ID, Event name, Activity ID, and Activity name columns define what event and activity the reminder belongs to.
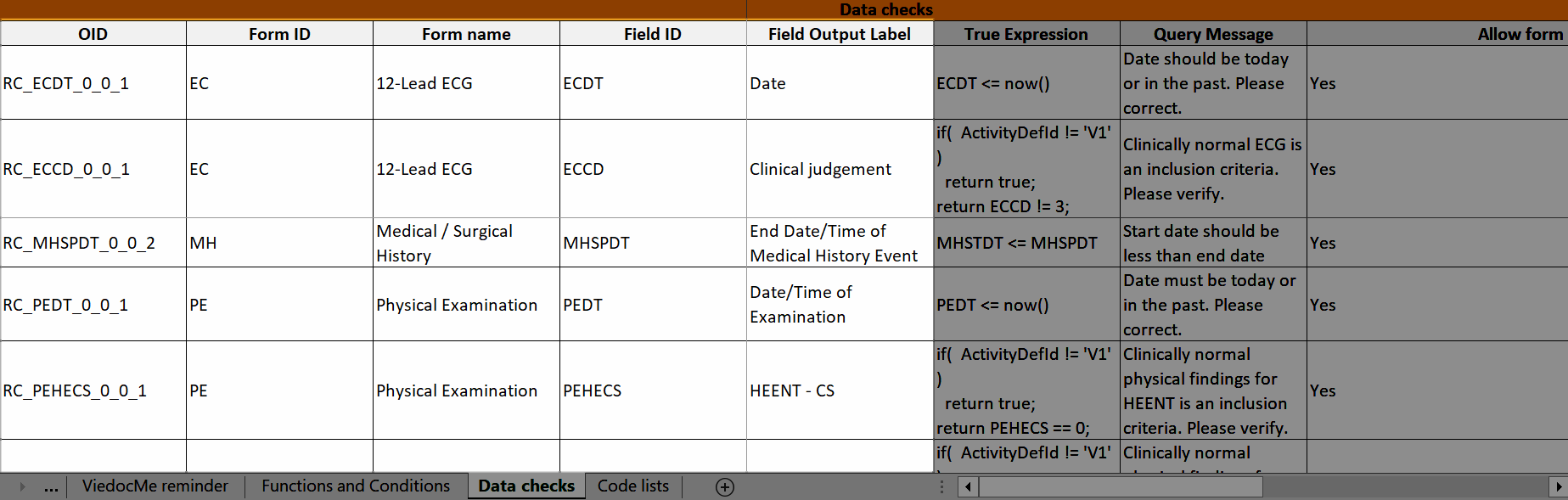
Data checks
The OID, Form ID, Form name, Field ID, and Field Output Label columns define the location of the data check.
Disclaimer: The overall structure of this report with regards to names and the order of columns can change to reflect future extensions of Viedoc Designer.
