Overview of Viedoc Logistics
Introduction
Viedoc Logistics is the interface for supply managers and all users with permission to manage the Investigational Products (IP) of a study. In Viedoc Logistics you can overview and monitor your IP (kits) as well as ship, receive and return kits between the depots and the participating sites.
Allocation of kits is managed from Viedoc Clinic and the feature is fully integrated with Viedoc Logistics, meaning that kits allocated by the site staff are instantly synchronized with and visible in the stock list.
System languages
Viedoc Logistics is available in the following languages:
- Chinese (Simplified and Traditional)
- English
- French
- German
- Japanese
- Portuguese
- Spanish
- Swedish
Access to Viedoc Logistics
A valid license is required to use Viedoc Logistics. Access is then given by a Study Manager or a Site Manager who invites the user to the study. For information on how to log in to Viedoc Logistics, see Launching Viedoc Logistics.
Overview of the interface
Viedoc Logistics consists of the following pages: Stock list (1), kit details view (2) and Study supply overview (3). The Study supply overview is only accessible for users with permission to manage kits on study level, this is also the landing page for those users. See Main functions below to read more about the interface.
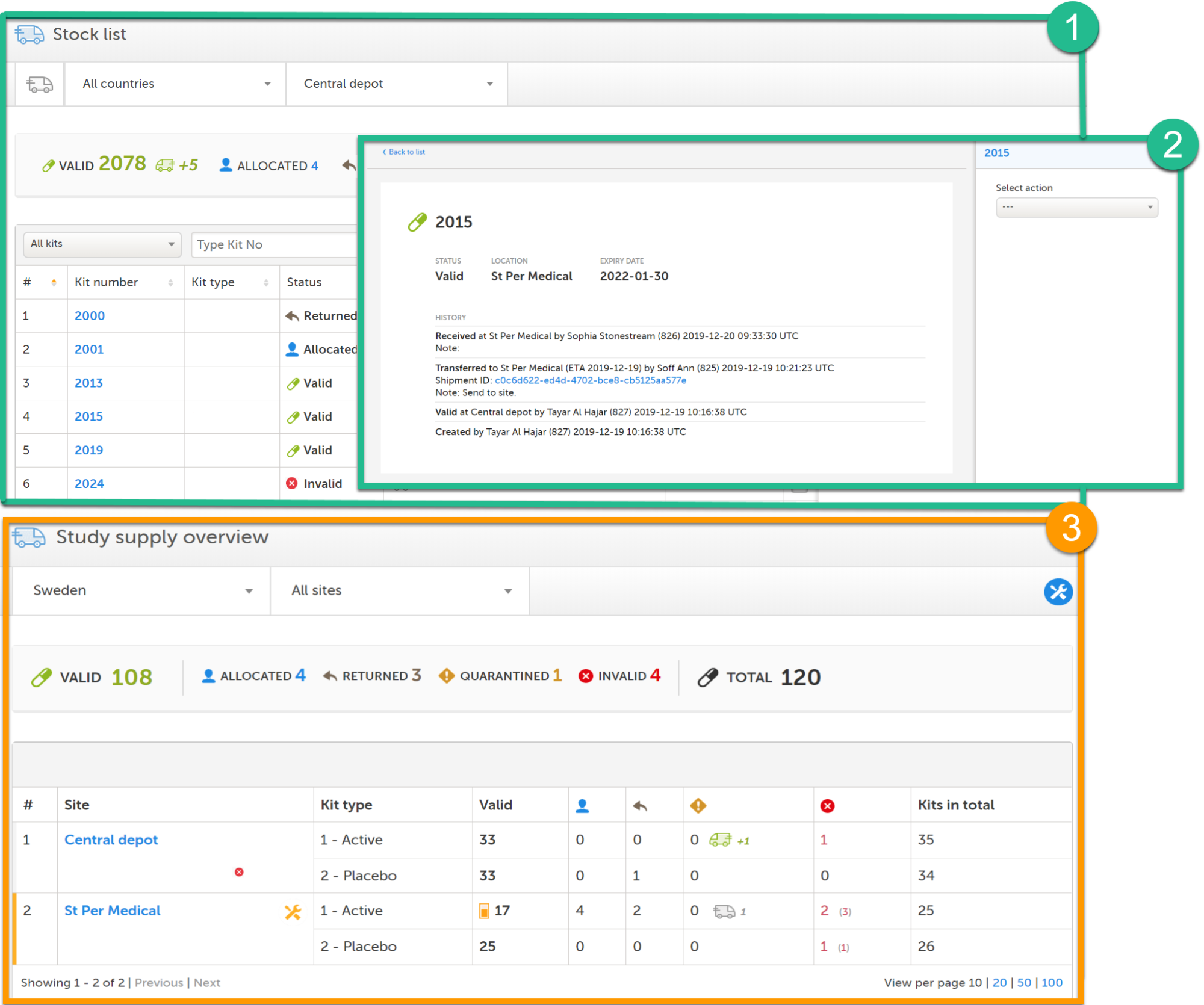
Scope of allocation
The scope of the allocation determines what depots and sites are enabled and used in Viedoc Logistics. The scope can be set to study, country or site level which is reflected in the interface as the available locations. Allocation of kits is also dependent on the scope, see section Scope of allocation in lesson Managing kits.
User roles
The user roles of Viedoc Logistics are configurable and the permission settings can be adjusted according to your study needs. There are two default roles:
- Site Supply Manager - for managing kits on site level
- Study Supply Manager - for managing kits on study level
Your permission settings determine what depots, sites and features are available to you. Usually, the Study Supply Manager monitors the supply for the whole study and transfers kits from the central depot to the participating (country) depots and sites. The Site Supply Manager usually manages the individual kits at the clinic (site).
Main functions
The following table summarizes the main functions of Viedoc Logistics:
| Page | Main functions | Accessible for users managing kits | Covered by lessons |
|---|---|---|---|
|
Stock list |
Transfer kits between depots and sites Set status to kits Track kits and shipments |
On site level (landing page) On study level |
|
| Kit details view | View audit trail of a kit Set status to a kit View shipment ID of a kit |
||
| Study supply overview |
Overview and monitor the study supply |
On study level (landing page) |
Terminology
For more terms, see our Glossary.
| Action | The activity that changes the kit status or location: Transfer - Receive - Cancel - Return - Invalidate - Quarantine - Restore - Edit expiry date |
| Central depot | The main supply depot of a study from where all kits are distributed. |
| Country depot | If the study is conducted in several countries, a country depot can be used as a distribution central to the sites within those countries. |
| ETA | Estimated Time of Arrival. The estimated day that a kit will arrive to destination when performing a transfer. The date is optionally added to the transfer and will be visible in the stock list and saved in the audit trail. |
| Global allocation list | The list of kits that is imported into Viedoc Logistics and constitutes the stock list in the Stock list page. |
| IP | Investigational Product. In Viedoc this is commonly named as kit. |
| Kit | The investigational product of a study. |
| Site supply manager | Default role for users managing kits on site level. |
| Shipment ID | The identification number that is created for each shipment. That is, for every transfer action a shipment ID is created and associated with the transfer. |
| Stock list | The stock list is the list of kits visible in the Stock list page, which is based on the global allocation list. The Stock list page is the landing page for site supply users. |
| Study supply manager | Default role for users managing kits on study level. |
| Study supply overview | The landing page for study supply users and interface for monitoring the study supply. |
