Tips & tricks
Unscheduled and common events
| Important! Unscheduled Events and Common Events are not available for PMS studies although they are visible on the Study workflow page. |
Adding Adverse Event forms
To add an Adverse Event form:
| 1 | Create an Adverse Event form with the form ID as AE and add it as a repeating form in a booklet. A form with an ID set to AE becomes an Adverse Event (AE) form. |
| 2 |
In AE Settings, on the General tab, set the ID of the AE form to AE. 
|
| 3 |
Set the format to be displayed in Viedoc Clinic in the Summary format field using the IDs of the items on the form. 
|
| 4 |
Select the pen icon on the form in the study workflow and select Allow form to repeat. This allows you to add multiple adverse events to a booklet. 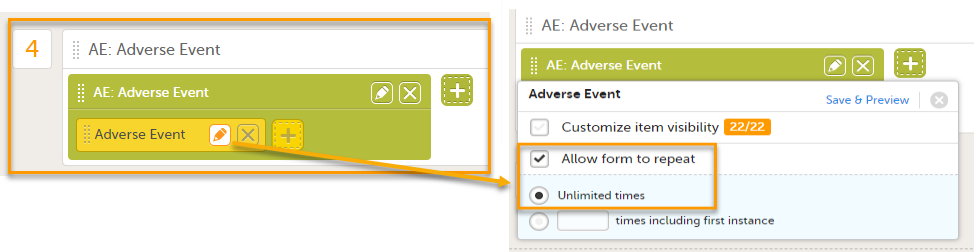
|
Note! All forms in the booklet, including the Adverse Event form, must be completed in order to submit the booklet. Therefore, the Adverse Event form should appear only when an adverse event occurs.
One way to implement this is described in the steps below:
| 1 |
Add a form to check if an Adverse Event occurred during the booklet period by adding the form AE occurred? 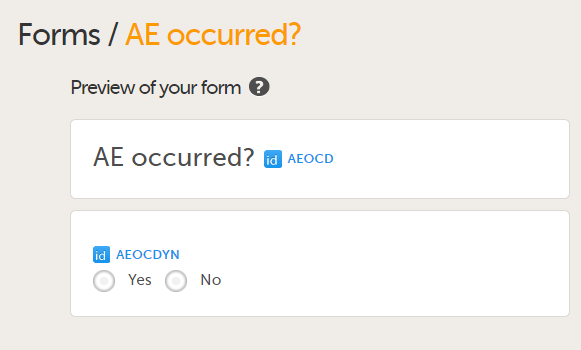
|
| 2 |
Configure the visibility condition of the Adverse Event activity using the added form item value. 
|
| 3 |
If you perform these steps, the Adverse Event form will become visible when an adverse event occurs, as shown in the example below. 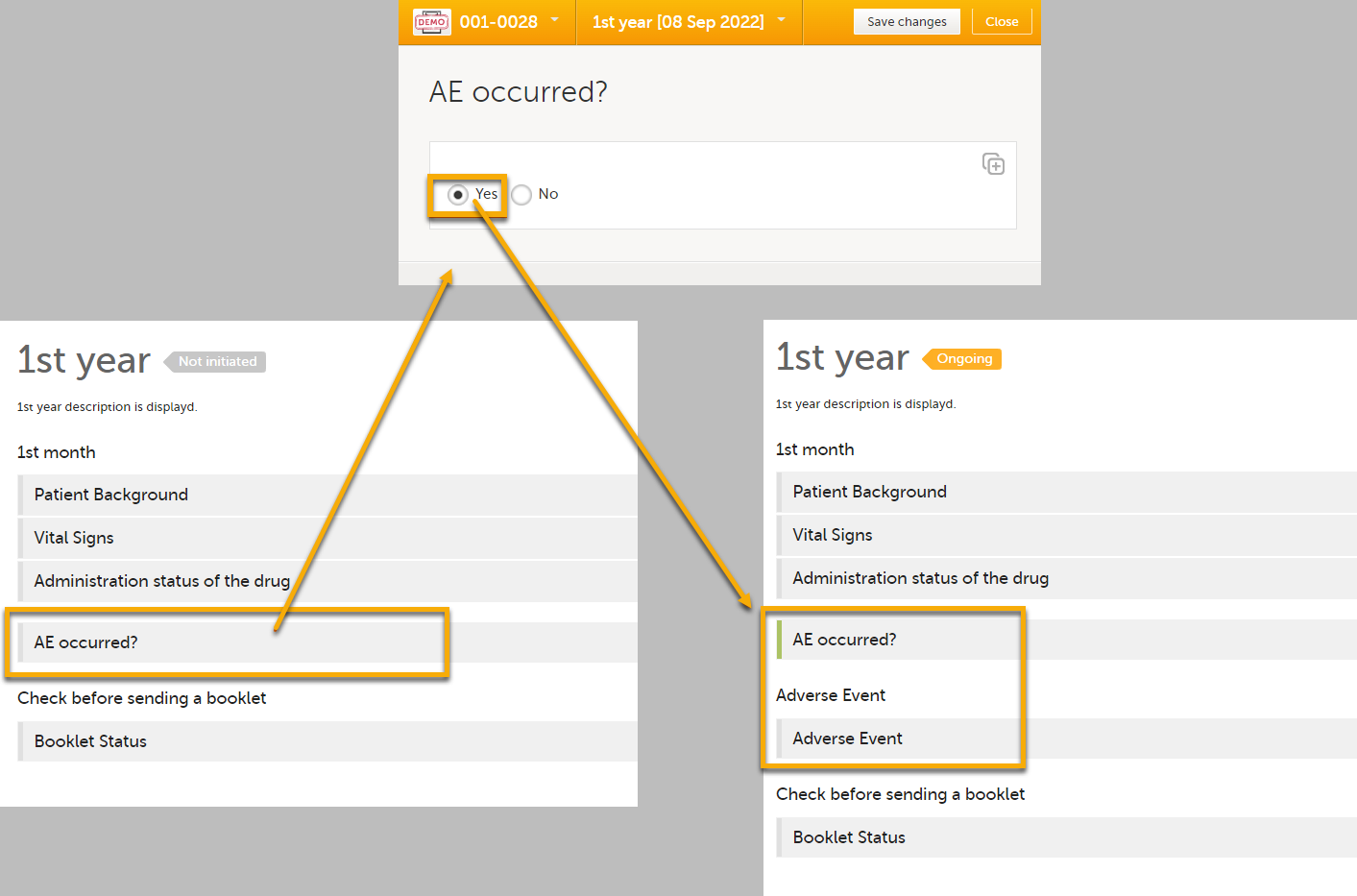
|
Managing Adverse Events independently
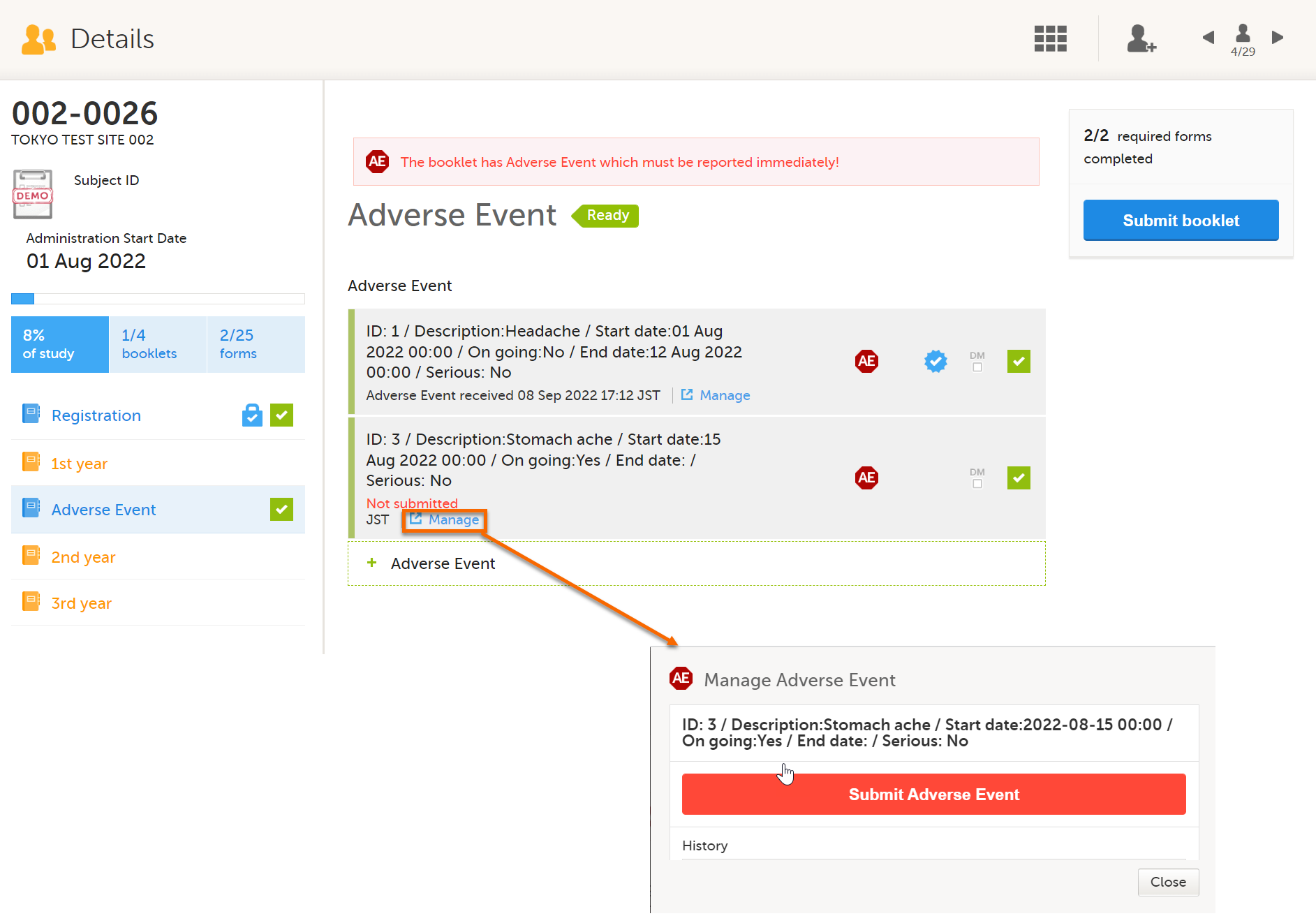
If you want to manage adverse events independently from other forms, you can do that by adding one booklet that contains only an AE form. You can report multiple adverse events at any date and time by selecting Allow form to repeat.

In Clinic, each time you add an adverse event, you can submit each adverse event individually.
You must submit by selecting Manage on the Adverse Event form. If you submit the booklet by selecting the Submit booklet button, the booklet is locked and you cannot add more adverse events.

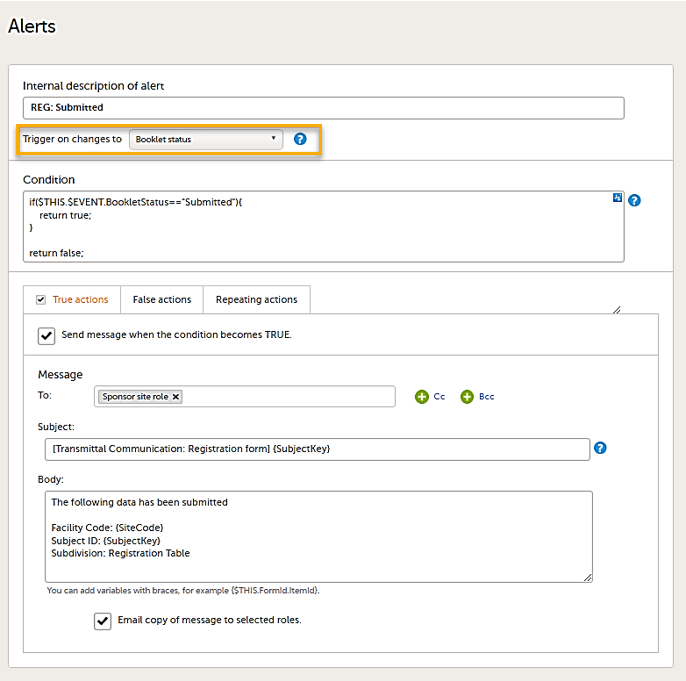
Setting alerts
You can add alerts to a study to notify users about important events.
Viedoc allows users to set up alerts that are issued under certain conditions (for example, when a severe adverse event occurs). It can be used to notify the Sponsor side that the Clinic side has submitted a booklet, or to notify the Clinic side that the Sponsor side has returned a booklet to request a re-investigation.
See Alerts for more information.
Setting up the alerts is done in Viedoc Designer, under Study Settings > Alerts:
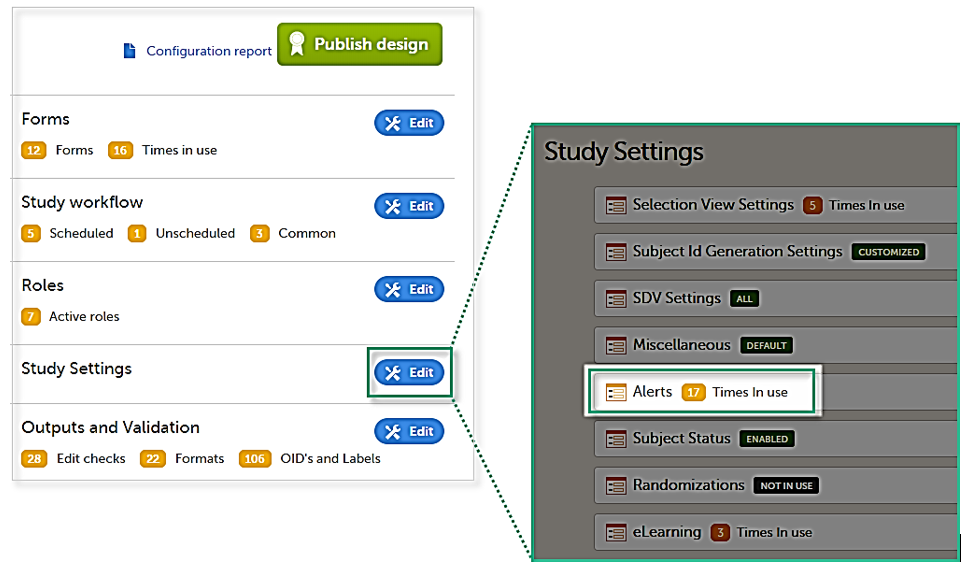
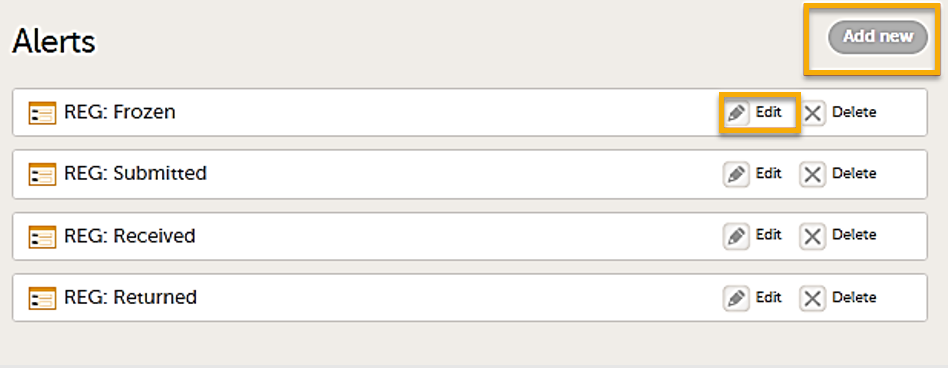
The Alerts page displays a list of existing alerts (if any) and allows you to add new ones, as illustrated below:
- The Add new button that allows you to create a new alert by opening the alert details page.
- The Edit button directs you to the alert details page, where you can see or edit the alert.
Alert triggers for PMS studies
On the Alert details page, you can define which type of change will trigger the alert. There are two options:
-
On context form data changes - the condition is evaluated when the selected context form below is saved. This option is the default for all existing alerts.
Sponsor users do not receive the message until the Sponsor side receives the booklets with settings that meet the alert condition.
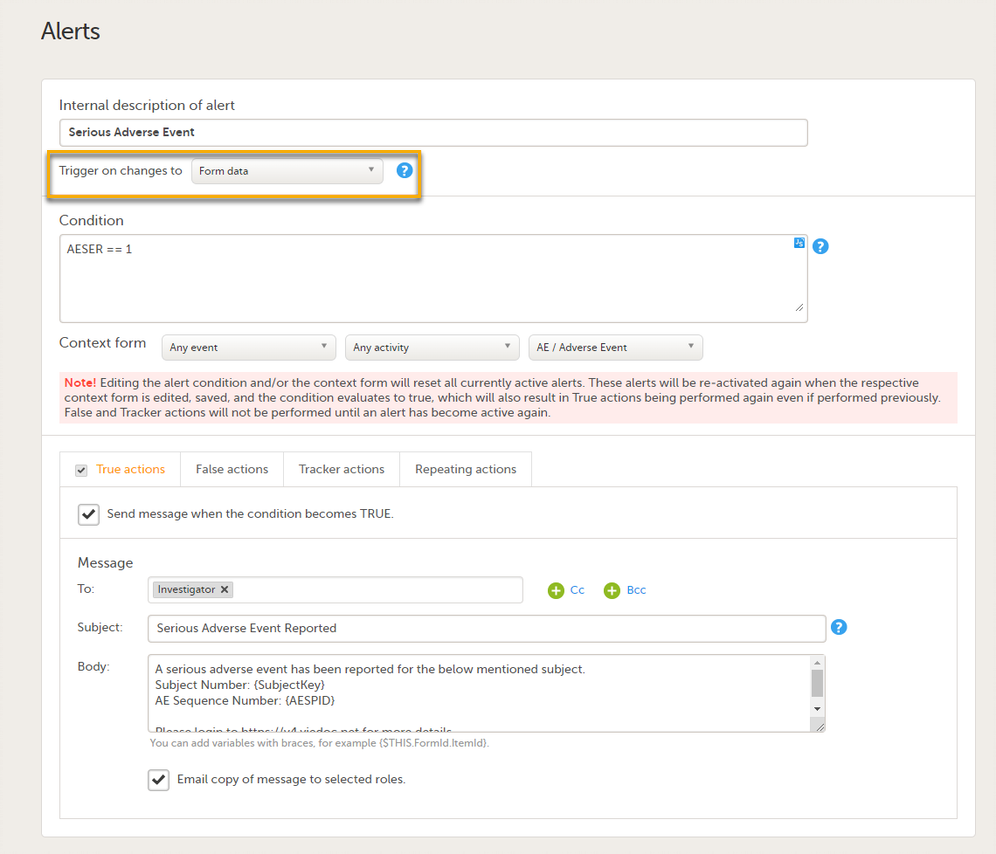
- On booklet status changes - the condition is evaluated when a booklet status is changed. The booklet that changes its status will then be the context.