Custom reports examples
Examples of Custom Reports built for Viedoc's template studies
Viedoc supports using the programming language R for custom reports. This lesson lists several custom reports written for our template studies or any studies that follow the Clinical Data Acquisition Standards Harmonization (CDASH) standards. For instructions about how to add custom reports, see Creating Custom Reports.
TIPS!
- Find downloadable example report scripts and practical R examples for visualizing and analyzing Viedoc data in the custom-reports repository in Viedoc's Github. The scripts can be customized adapted to your study’s needs.
- You can also download a zip file containing the R code for the individual reports here.
Details and example images of each report are shown below:
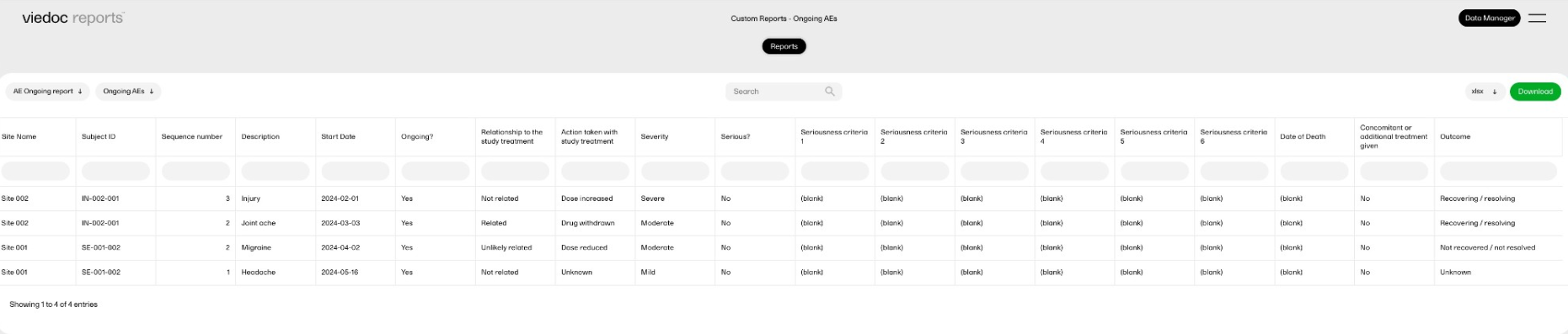
Ongoing adverse events report
This report displays all ongoing adverse events. This demonstrates a good example of how to filter data based on specific criteria, as well as how to create a report with two sub-reports.
This custom report generates the following output:
-
Sub-report 'Ongoing AEs': A table of all adverse events (AEs) that are ongoing, sorted by start date (ascending).
-
Sub-report 'Start Date > 30 days': A table of ongoing AEs with a start date of more than 30 days ago.
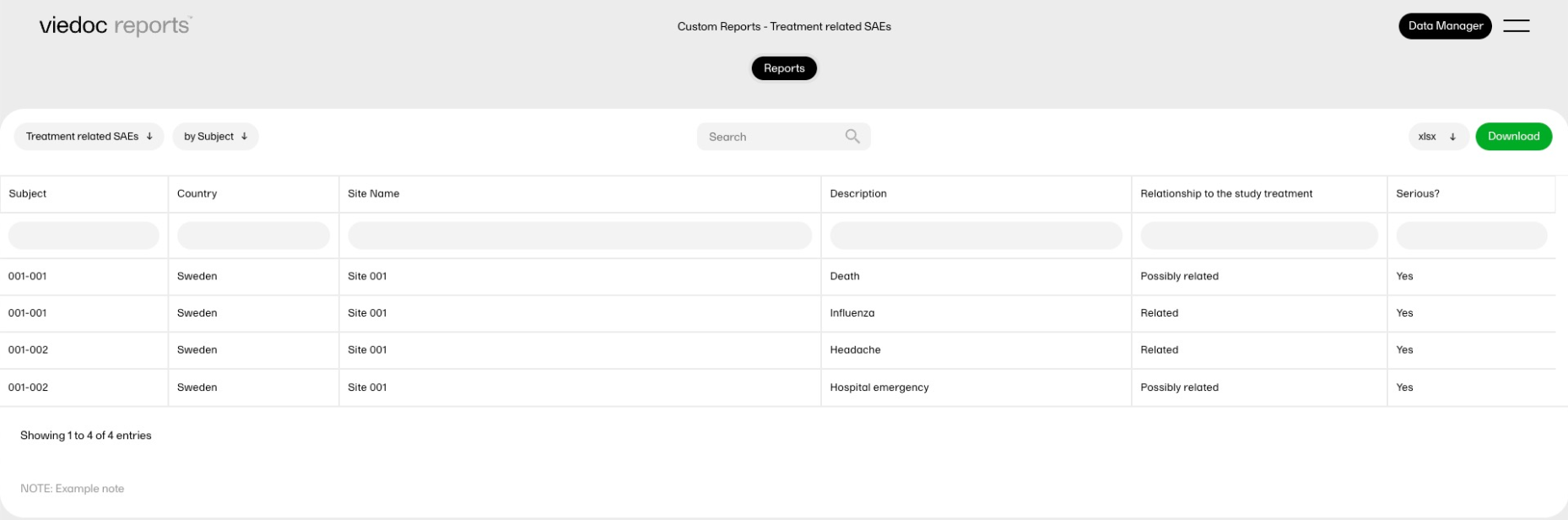
Treatment-related serious adverse events report
This report displays selected data consisting of adverse events (AEs) that were recorded as treatment-related and serious, and summarizes the data by site.
This custom report generates the following output:
-
Sub-report 'by Subject': A table of all AEs entered as possibly related to the study treatment and as Serious.
-
Sub-report 'by Site': A table of the number of AEs fulfilling the above criteria per site.
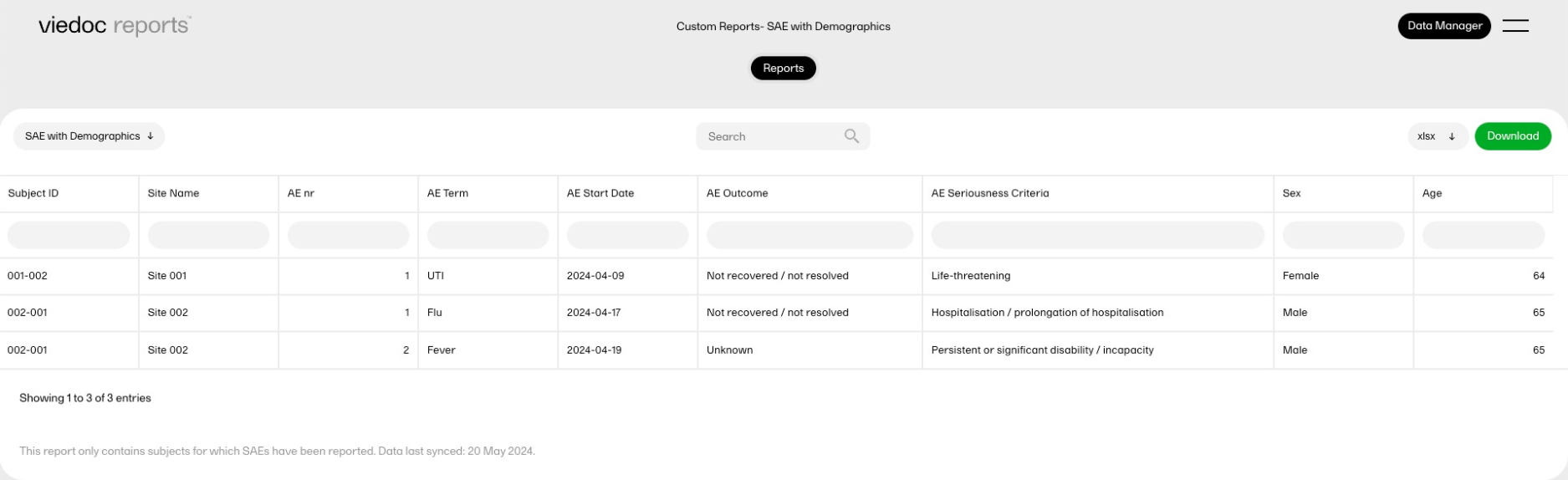
Serious adverse events combined with demographic data
This report displays all the serious adverse events (SAEs) with the corresponding demographic data. It is an example of how data from two different forms can be combined into a single custom report, as well as flag missing data.
This custom report generates the following output:
-
A table of AEs entered as Serious, combined with the subject's sex and age from the demographic form.
Outliers
This report displays statistical outliers identified in the data.
This custom report generates the following output:
-
Sub-report 'Systolic BP': A table listing outliers in the systolic blood pressure data.
-
Sub-report 'Diastolic BP': A table listing outliers in the diastolic blood pressure data.
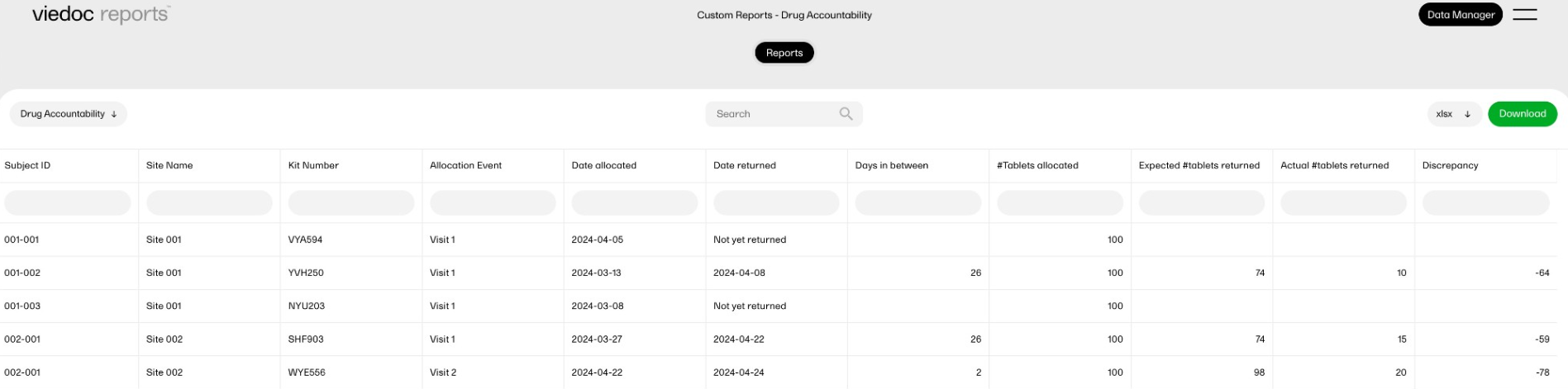
Drug accountability
This report calculates the drug accountability between two visits, and displays the calculated values in new columns. It demonstrates how a custom report could be used to calculate scores or other metrics.
This custom report generates the following output:
-
A table of allocated and returned kits with the expected and the actual returned numbers of tablets.
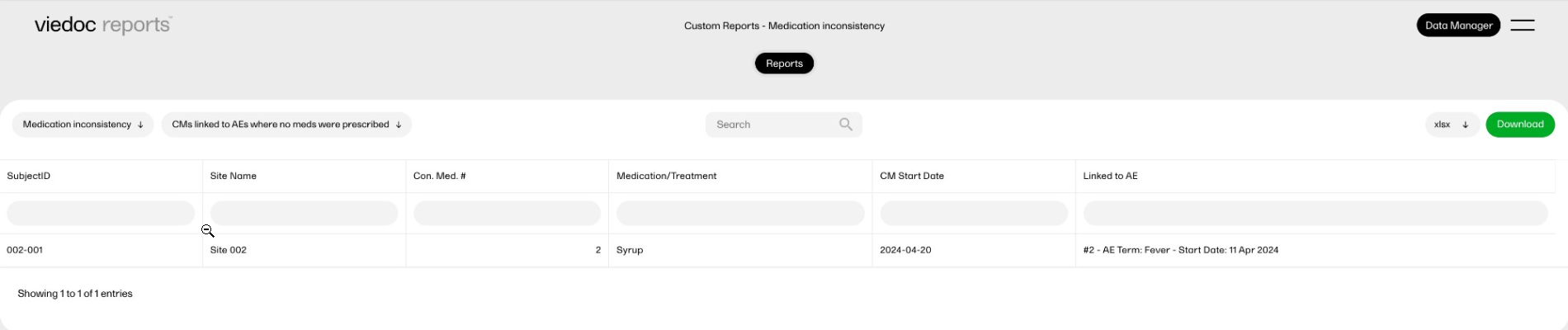
Medication inconsistency
This report compares AEs with concomitant medication (CMs) to check for inconsistencies in data entry. This is something that previously was an offline check that required a manual comparison of the data. This custom report provides a list of the problematic data immediately.
This custom report generates the following output:
-
Sub-report 'CMs linked to AEs where no meds were prescribed': A table showing the concomitant medication (CMs) entries that are linked to the adverse events entries in which it was reported that no treatments or medications were prescribed.
-
Sub-report 'AEs where meds were prescribed not linked to CMs': A table showing adverse events entries for which it was reported that treatments or medications were prescribed, but for which no concomitant medications entry exists.
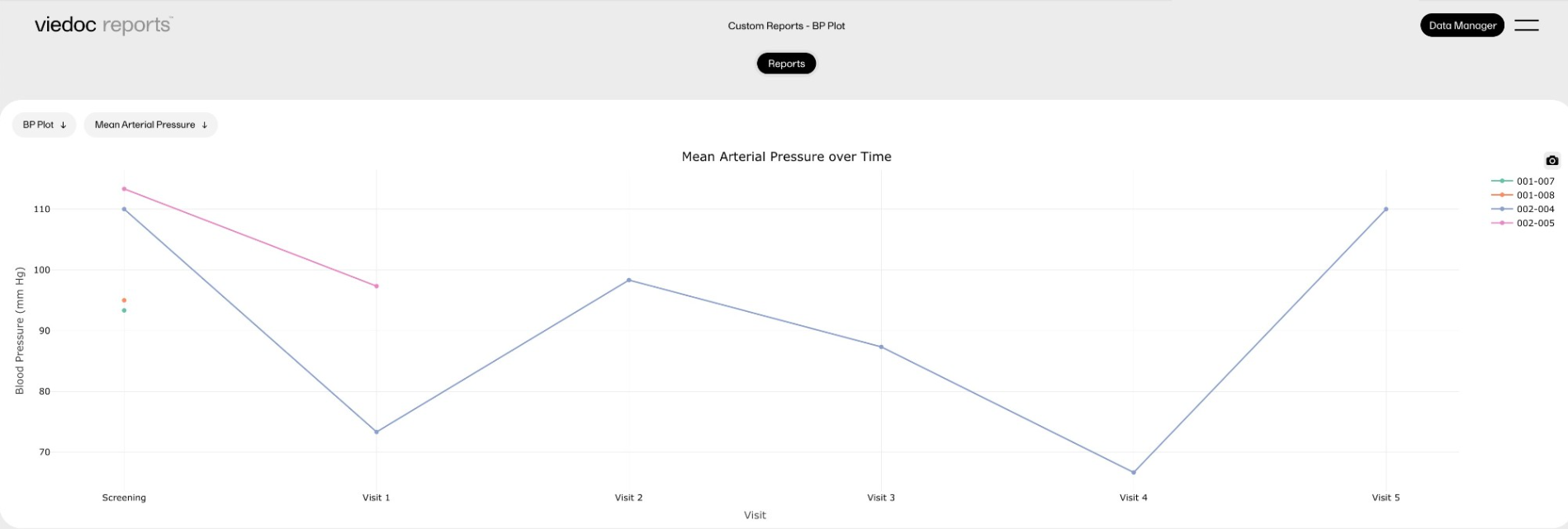
Blood pressure plot
This report displays simple scatter plots using the 'plotly' package.
This custom report generates the following output:
-
Sub-report 'Mean Arterial Pressure' (MAP): A plot of the calculated MAP.
-
Sub-report 'Systolic only': A plot of the systolic blood pressure.
-
Sub-report 'Diastolic only': A plot of the diastolic blood pressure.
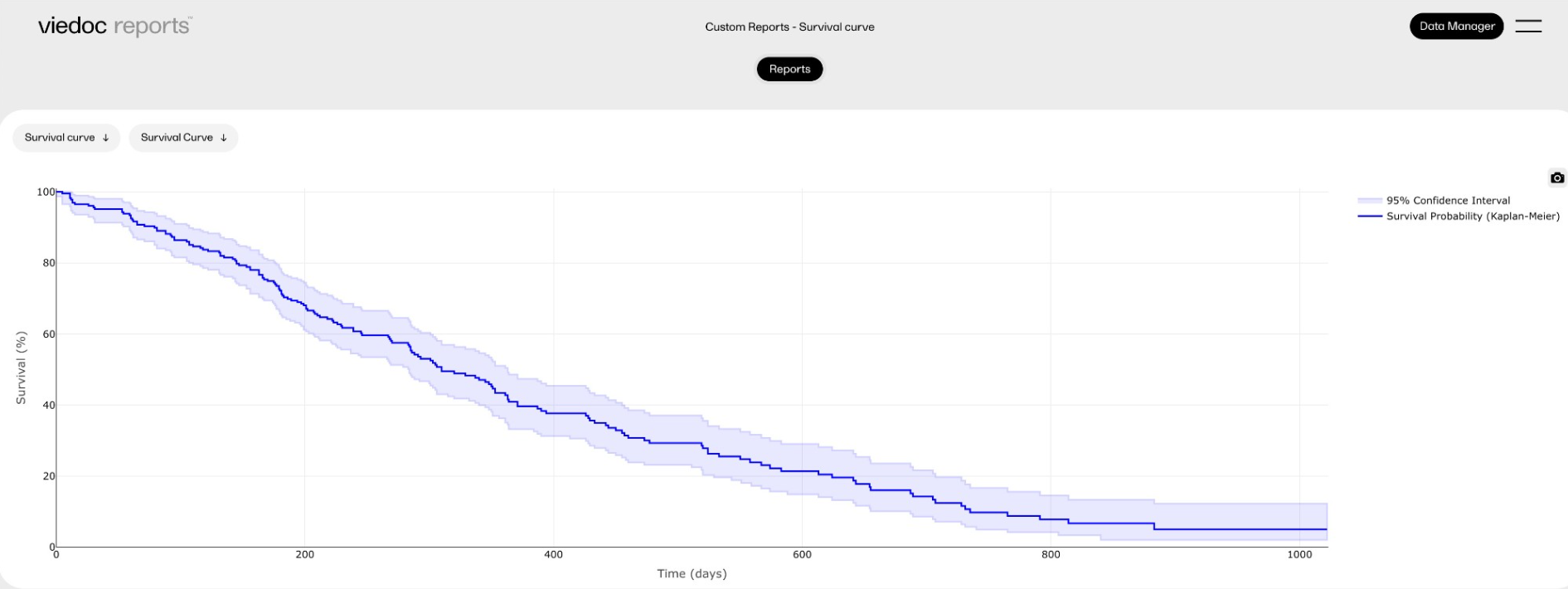
Survival curve
This report displays a survival analysis using the Survival package, as well as a more complicated plot using the 'plotly' package.
This custom report generates the following output:
-
Sub-report 'Survival Curve': A plot of the Kaplan-Meier model, with 95% confidence intervals.
-
Sub-report 'Survival Table': A table with the plotted values.