Migrating a study design from training to production
Introduction
This lesson provides an overview of the test and production servers and describes the main steps to be performed when building a study on the training server and then migrating it to the production server.
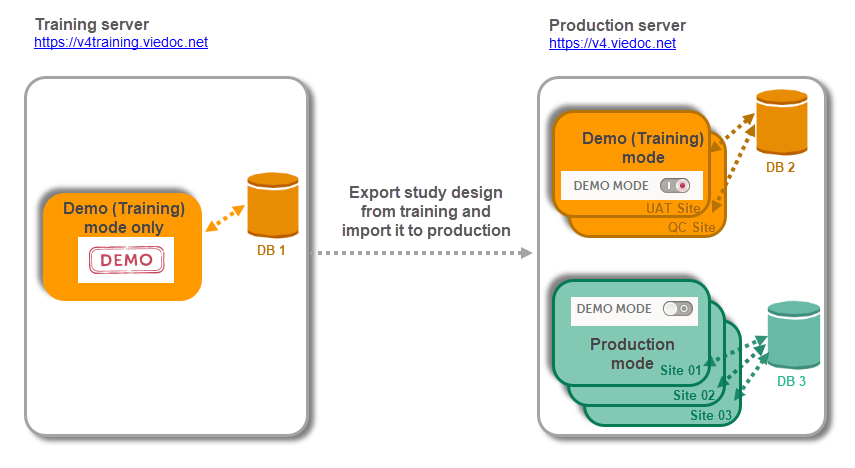
Please note that there are different database instances the data is saved on depending on the server (i.e. training server and production server), as well as depending on the operation mode (i.e. Demo (Training) mode and Production mode).
Training server
As a Viedoc client, you will be provided first with access to the so-called training server (v4training.viedoc.net). The purpose of the test/build server is to allow you to evaluate and use Viedoc without the need of a contract for a specific study. No license (Reference ID) is required for this server. Here you can build a study and perform all kinds of tests, with all the sites running in demo mode.
Note! It is not guaranteed that studies running on the test/development server are completely and continuously backed-up. This server should therefore never be used for any production studies.
Production server
Any study that is supposed to be taken in production is normally initiated on the training server and later moved to the production server (v4.viedoc.net) once it is “ready” to be shared with the Sponsor or other external party.
Studies and/or study designs can be easily transferred from one server to the other via the Clinical Data Interchange Standards Consortium (CDISC) Operational Data Model (ODM) export and import feature in Viedoc Designer. For detailed instructions see the step by step guide below.
For a study on the production server it is possible to configure the sites to operate in one of the following:
- training(demo) mode only
- production mode only
- both training (demo) and production modes (not recommended, see section Training(Demo) vs Production mode below).
|
Important! The demo mode of a production study should not be confused with a study on the test/build server. When a study has sites of both production and training types added, a switch will be available in Viedoc Clinic, making it possible to choose in which mode the data will be entered to, that is, demo or production. |
Training(Demo) vs Production mode
When the study is completely set up in the production environment, there are two different modes that a site can be set to operate on, as described below. This is configured in Viedoc Admin under Site Settings (see image below and detailed instructions in Managing study sites lesson). There are two different database instances that the data will be saved on for each of the modes, that is, when operating on demo mode the data will be saved only on the demo database instance and when operating on production mode, the data will be saved only on the production database instance.
- Training (demo) mode only - does not require a license, and the data will be saved on the demo/training instance only. This is to be used for the test sites only.
Important! As data entered when operating in this mode is saved on a separate database instance, this should never be used for entering any real data, but for testing purposes only. - Production mode only - this is used for the production sites, i.e. real sites where real data will be entered, not for testing purposes.
Important! A valid license (Ref ID) is needed in order to be able to set a site to operate in production mode. - Both training (demo) and production modes - This is possible only if the Allow single sites to be in both modes (production and training mode) option is selected in Viedoc Admin under Study Settings. A switch will be available in Viedoc Clinic, making it possible to choose in which mode the data will be entered to, i.e. demo or production.
Important! This is not recommended, because setting the same site to operate in both Production and Training mode: - might cause real data to be saved on the demo database instance and demo/test data to be saved on the production database instance if the Demo mode switch in Viedoc Clinic is not used correctly.
- would make it difficult to remove the access of a user only to the demo or production mode. A user having access to a site operating in both modes will always have access to the site in both demo and production.
Given the above described functionality, it is recommended, on the production server, to have separate site(s) only in Training (demo) mode, for testing/demo purposes (for example, for User Acceptance Testing (UAT)), and the production sites to operate only in Production mode. This way, the user access can be easily managed and the risk of mixing real data with test/demo data is eliminated.
Step by step guide - migrating a study from training to production
| 1 |
Build and test your study on the training server. |
| 2 |
Export the study design, as described in Exporting/Locking/Deleting a study design. Notes! The exported study design does not contain the Global design settings and Viedoc Me translations. These will need to be performed again manually on the production environment after the design is imported. See the next step for details. |
| 3 |
On the production server:
|
| Important! This process cannot be used for revising an existing design version on production, as importing the design will always result in a totally new version. |
