Configuring medical coding scopes
This lesson describes how to configure medical coding scopes in Viedoc Designer.
Introduction
About medical coding
Viedoc offers support for medical coding. The medical coding feature allows you to code data, such as Adverse Events, Medical History and Concomitant Medications, in a standardized way.
Viedoc supports the following types of dictionaries:
- Medical Dictionary for Regulatory Activities (MedDRA) (including Chinese version)
- MedDRA/J (Japan)
- Anatomic Therapeutic Chemical Classification System (ATC) without Defined Daily Dose (DDD)
- Iyakuhinmei Data File (IDF)
- World Health Organization Drug Dictionary (WHO DD) WHODrug - C and C3 formats (including Chinese version)
Workflow
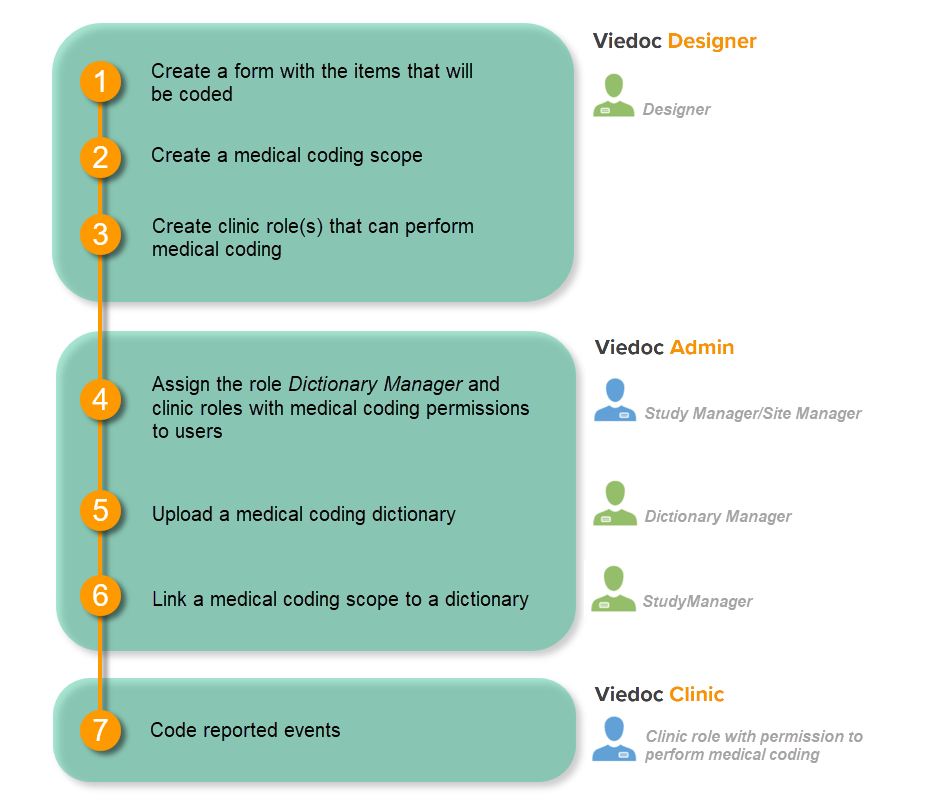
Medical coding is configured in Viedoc Designer and Viedoc Admin, and executed in Viedoc Clinic. The schematic below depicts what different steps need to be taken, and which roles have permission to perform these steps.
This is a single-sourced file that should have the following content:
Introduction to medical coding, description of the workflow.
For more detailed instructions regarding these steps, see:
- Configuring medical coding scopes in Viedoc Designer (this lesson!)
- Managing medical coding dictionaries in Viedoc Admin
- Medical coding in Viedoc Clinic
Medical coding scopes
What is a medical coding scope?
A medical coding scope maps the items to be coded to a medical coding dictionary. The scope defines the following:
- which item in which form should be coded,
- at what level the coding should break, in case data in the form are changed (see Breaking the medical coding), and
- the type of dictionary that should be used for coding.
After a medical coding scope has been created in Viedoc Designer, and linked to a medical coding dictionary in Viedoc Admin, the defined items become available for coding in the medical coding console in Viedoc Clinic.
You need to create a separate scope for each item to be coded. Only text items can be coded. If you would like to code the same item multiple times using different types of dictionaries, you need to create a separate scope for each dictionary type to be used for that item.
Breaking the medical coding
The medical coding of already coded items will break when data in, or related to, these items is edited. In the medical coding console in Viedoc Clinic, these items will be flagged and need to be re-coded. It is therefore necessary to define when the medical coding should break. In other words, it is necessary to define the level at which editing of the data should lead to breaking of the code. For example, if form level is selected, the coding will break when any item in the form that contains the coded item is edited. If item level is selected, the coding will only break if the coded item is edited.
The level at which medical coding breaks is determined in the medical coding scope in Viedoc Designer. It is possible to select one of the following levels for breaking the code:
- event
- activity
- form
- item group
- item
Step-by-step guides
Workflow in Viedoc Designer
In order to set up medical coding in your study, you have to perform the following steps in Viedoc Designer:
- Create a form with the items that should be coded. See the eLearning section Creating and editing forms.
- Create a medical coding scope. See Creating a medical coding scope below.
- Create a clinic role that can perform and approve medical coding. See Creating a clinic role that can perform and approve medical coding below.
Creating a medical coding scope
To create a medical coding scope, follow the steps below.
| 1 |
In Designer, scroll to the study for which you would like to create medical coding scopes, and click the Edit icon in the Global design settings field to open the Global design settings window. |
| 2 |
In the Medical coding field, click the Edit icon to open the medical coding settings. 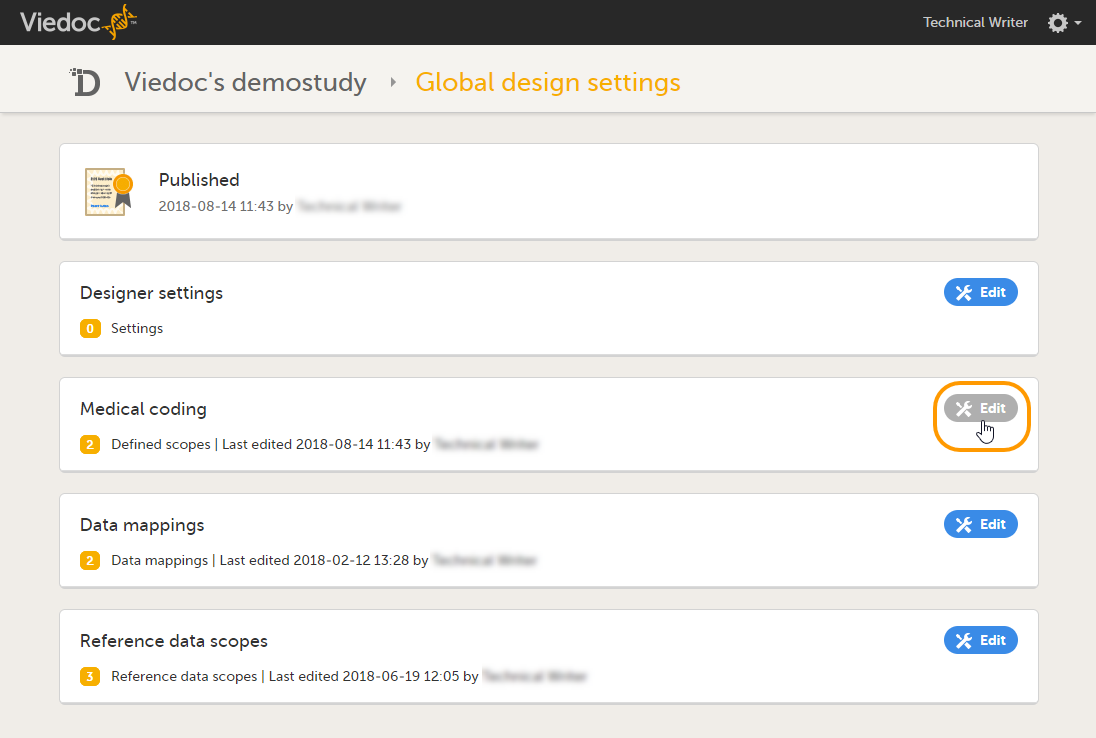
|
| 3 |
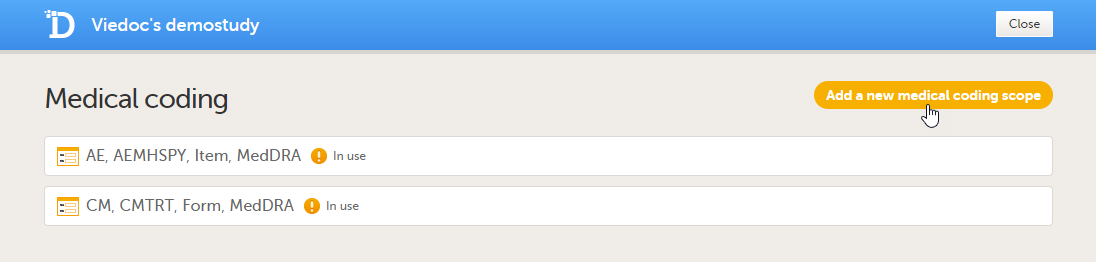
Click Add a new medical coding scope.
|
| 4 |
Set-up the scope:
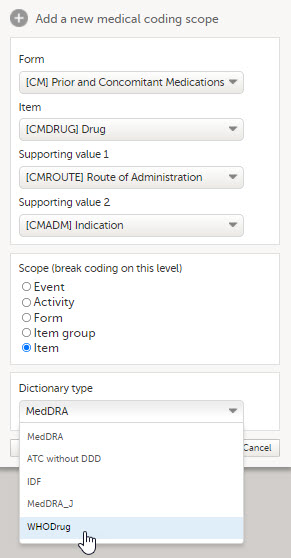
|
| 5 | Click Ready. The pop-up closes. |
| 6 | Click Save changes, and click Close to close the Medical coding window. |
Note! For the medical coding scope to take effect, you need to publish the global design settings.
Creating a clinic role that can perform and approve medical coding
In order to use medical coding in your study, you need to set up one or more clinic roles that have the following permissions (rights):
- Medical coding, together with
- Perform medical coding, and/or
- Approve medical coding.
Note! You need to have at least one clinic role with permission to perform medical coding.
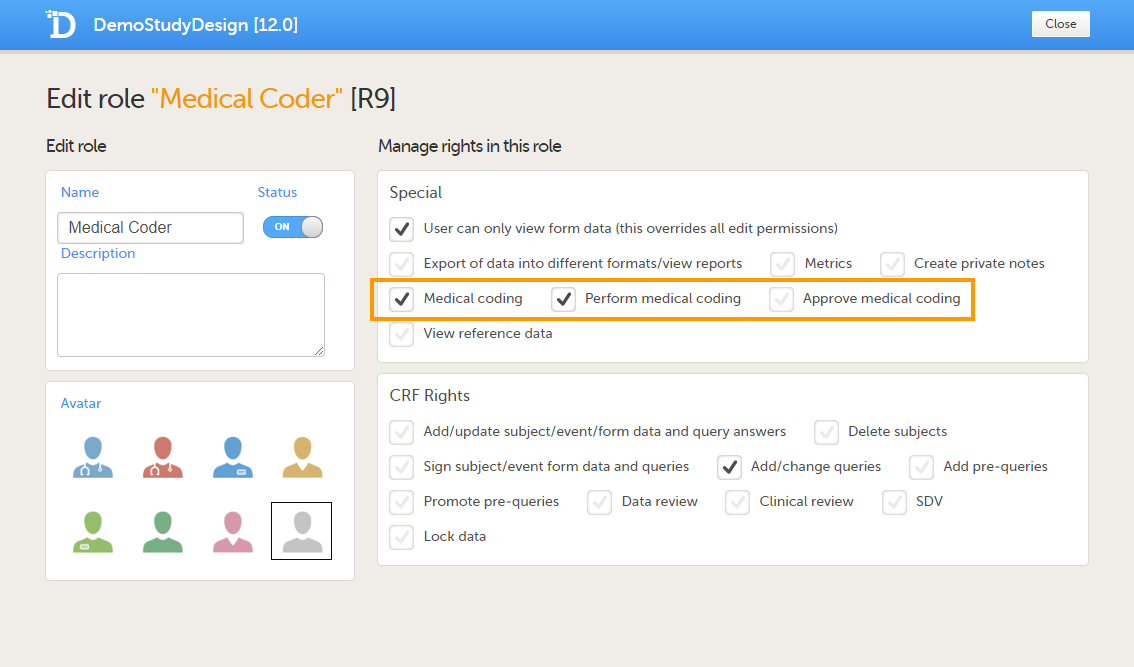
Roles that only have the permission Medical coding activated, without Perform medical coding or Approve medical coding, can see the medical coding status in Viedoc Clinic, but cannot open the medical coding console and perform or approve medical coding. This setting is typically used by a project manager or a sponsor.
The permissions (rights) for each clinic role are configured in the Roles section of the study design In Viedoc Designer. For more detailed instructions on how to configure clinic roles, see Configuring roles.
Editing a medical coding scope
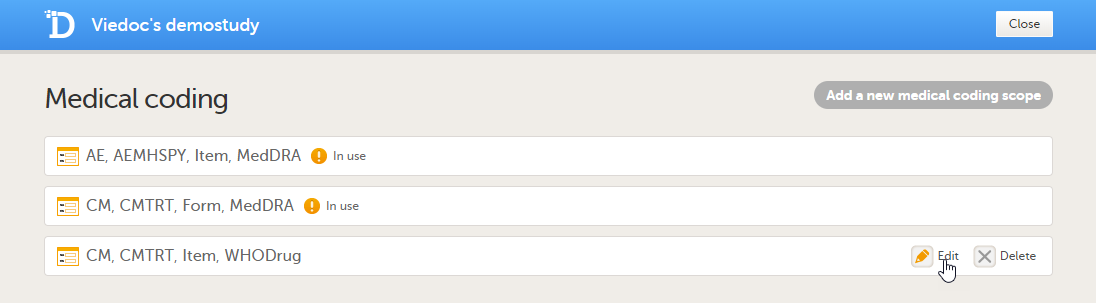
You can edit a medical coding scope by clicking Edit.
Note! It is not possible to edit a medical coding scope when the Global design settings have been published.
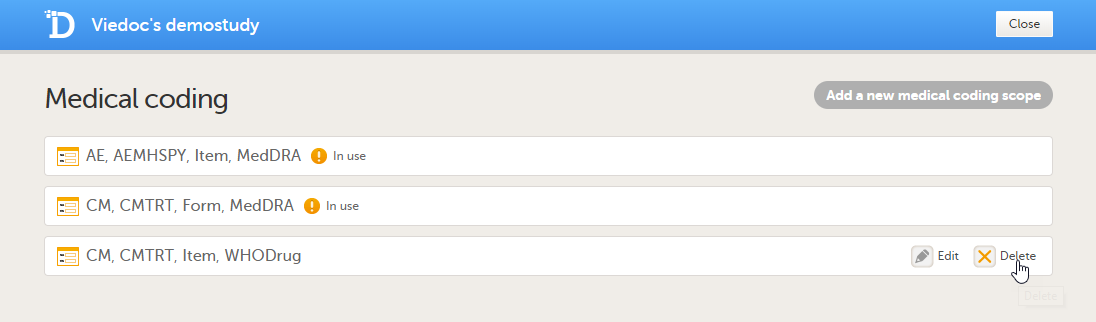
Deleting a medical coding scope
You can delete a medical coding scope by clicking Delete.
Note! It is not possible to delete a medical coding scope when the Global design settings have been published.