Introduction to Viedoc eTMF
Overview
Viedoc eTMF is a digital repository for capturing, managing, sharing, and storing essential documents for your clinical trial.
Viedoc eTMF is based on the TMF Reference Model by the Drug Information Association (DIA). The TMF Reference Model is an industry consensus catalog of all TMF records. Using the TMF Reference Model ensures compatibility and interoperability with other clinical trial parties, such as CROs.
The TMF Reference Model includes documents in all different phases of a clinical trial:
- Before the start of the trial
- During the trial
- After study termination
The TMF Reference Model categorizes documents in zones, sections, and artifacts in a hierarchical structure.
The set of zones, sections, and artifacts included is defined in a template file that is maintained by the eTMF Manager.
The TMF can include both the Investigator Site File (ISF) and the sponsor TMF.
For portability reasons, the DIA TMF Reference Model is defined in an Excel file.
Viedoc eTMF also uses Excel files as templates for the eTMF structure.
Roles and permissions
The user access to Viedoc eTMF is determined by the assigned roles and permissions. eTMF roles and permissions can work in combination or independently.
eTMF roles
These roles are defined in the template, which is maintained by the eTMF Manager.
Depending on the permission associated with your user role, you can perform different actions on documents. Your user role can have permission (no access, read, write, or review) on these TMF levels:
- Study/trial
- Country
- Site
You can only see and access documents if you have permissions for the artifact on the corresponding TMF level.
For example, if an artifact is linked to two sites, a user with write permission for the artifact for only one of the sites will be able to read but not edit the document. This is due to the fact that the user does not have write permissions for all sites that the document is linked to.
eTMF permissions
Note that this text section is available also in the lesson Overview of Viedoc eTMF.
The permissions are defined in Viedoc Admin and are assigned to you by the eTMF Manager.
The eTMF permissions are:
- Archive sponsor TMF
This permission gives the mapped user role access to the TMF Archive view and the ability to archive artifacts that are listed as Sponsor side (set in the Edit artifact window or in the template file on the sheet V 3.1.0, column M Sponsor Document). This is used for creating the main archive of the study documents. - Archive investigator TMF
This permission gives the mapped user role access to the TMF Archive view and the ability to archive artifacts that are listed as Investigator side (set in the Edit artifact window or in the template file on the sheet V 3.1.0, column N Investigator Document). This is used for creating/archiving an Investigator Site File. - Read-only TMF Admin
This permission gives the mapped user role the ability to inspect the structure, templates, and other settings in the TMF Admin view in read-only mode.
A user with this permission can access the TMF Admin view and is able to:- View a selected/instantiated structure
- Export templates and structure
- View the settings tab
- Read-only Trial Master File
A user role with this permission will gain read access to all the published documents in the Trial Master File view. If this permission is assigned in combination with an eTMF role, the no access permission, set in the template file for that specific role, will be overridden by read access by the system. - Download audit trail
A user role with this permission will be able to access the TMF Archive view and generate the complete audit trail report from there. - Manage drop zone
This permission gives the mapped user role access to manage the files in the shared drop zone.
Note! For more information about permissions and accesses, see eTMF access use cases.
Document statuses and actions
The following image shows the document version statuses and the actions that change the status of a document version. The initial status of a document when it is uploaded to the eTMF is Unpublished.
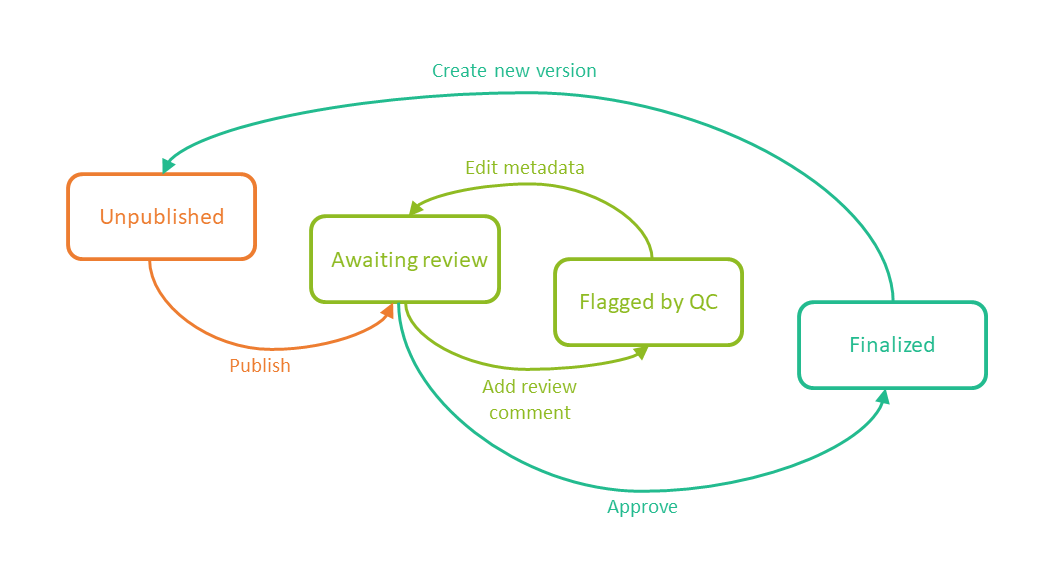
If you edit metadata for a document version that is Unpublished or Awaiting review, the document version status is not changed.
It is not possible to edit the metadata of a Finalized document. To make changes, a new version needs to be created.
Note! Different actions require different permissions, which means that they are performed by users with different user roles.
eTMF user view
The eTMF user view is your starting point for working with documents:
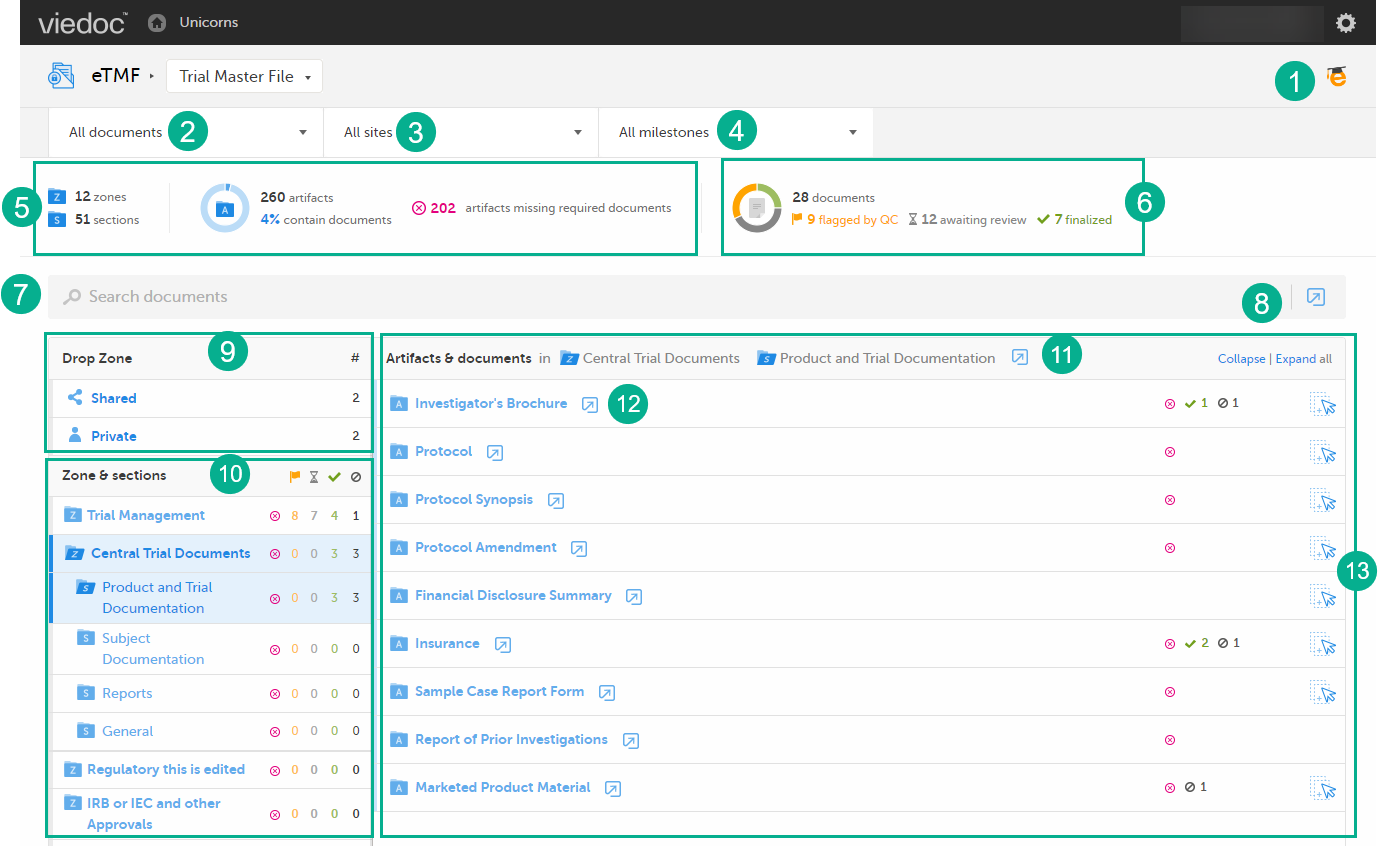
1. Link to the eLearning curriculum Viedoc eTMF User Guide
2. Use the dropdown menu to filter your view by Trial level or Country level. You can only select a country that you have permission for.
3. Use the dropdown menu to filter your view by site. You can only select a site that you have permission for.
4. Use the dropdown menu to filter the artifacts by milestones. You can either select a milestone group (Start Up, Study Conduct, Close Out, Other) or a specific milestone that is defined in the structure. Selecting a group means filtering the artifacts by all the milestones that belong to that group.
5. The left eTMF metrics area gives an overview of the published documents for the complete trial.
6. The right eTMF metrics area gives an overview of the published documents belonging to the trial artifacts that you have access to.
7. Use the search field to search for words or sub-strings in filenames or within file content.
8. Click the Details page button to open the Details page, listing all eTMF documents. For more information, see The Details page.
9. Use the Drop Zone area to upload files to be managed later either by you or by the drop zone manager.
10. Use the Zones & sections area to navigate through the eTMF structure. Click on zones and sections to expand/collapse them.
11. Click the Details page button to open the Details page, listing the documents in the section. For more information, see The Details page.
12. Click the Details page button to open the Details page, listing the documents in the artifact. For more information, see The Details page.
13. The Artifacts & documents area is where you can view documents and their status. In this area, you can also upload and download documents, delete unpublished documents, as well as open the Document properties dialog.
Note! All date and time stamps in the Trial Master File view are according to the user's local time zone.
