Archiving a study
Introduction
When data collection at a study site has been confirmed and completed, each site should export and archive the study data and site-related documentation.
Prerequisite
Site users must have the role permission to export data for the sites where the archiving should be performed. For more information, see the Data export lessons in Viedoc Clinic User Guide.
If Viedoc eTMF is used, see the following lesson eTMF-EMS repository.
Archiving the study
The following documentation is recommended to export when archiving a study at site. Export of data is still possible for locked studies.
- The user logs (available in PDF and Excel). For more information, see the User logs section in Study start page.
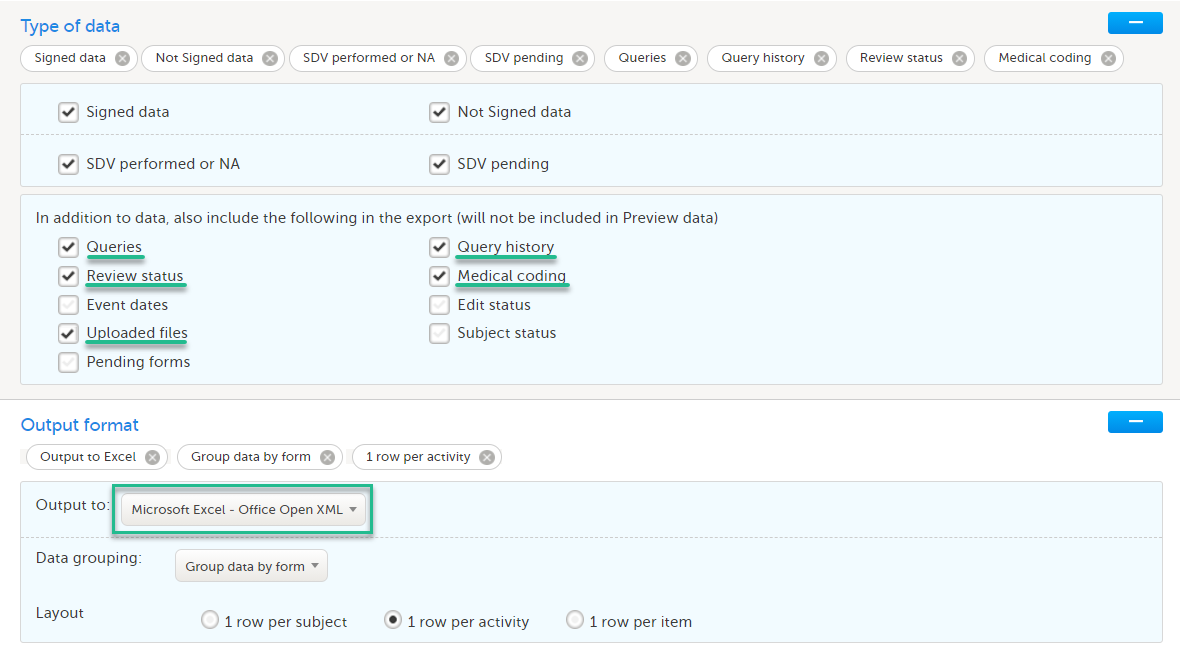
- The CRF data in all available formats (including all visits and forms). For each format, it is advisable to select the following:
- Excel
- Queries, Query history, Review status, Medical coding, Uploaded files
- Queries, Query history, Review status, Medical coding, Uploaded files
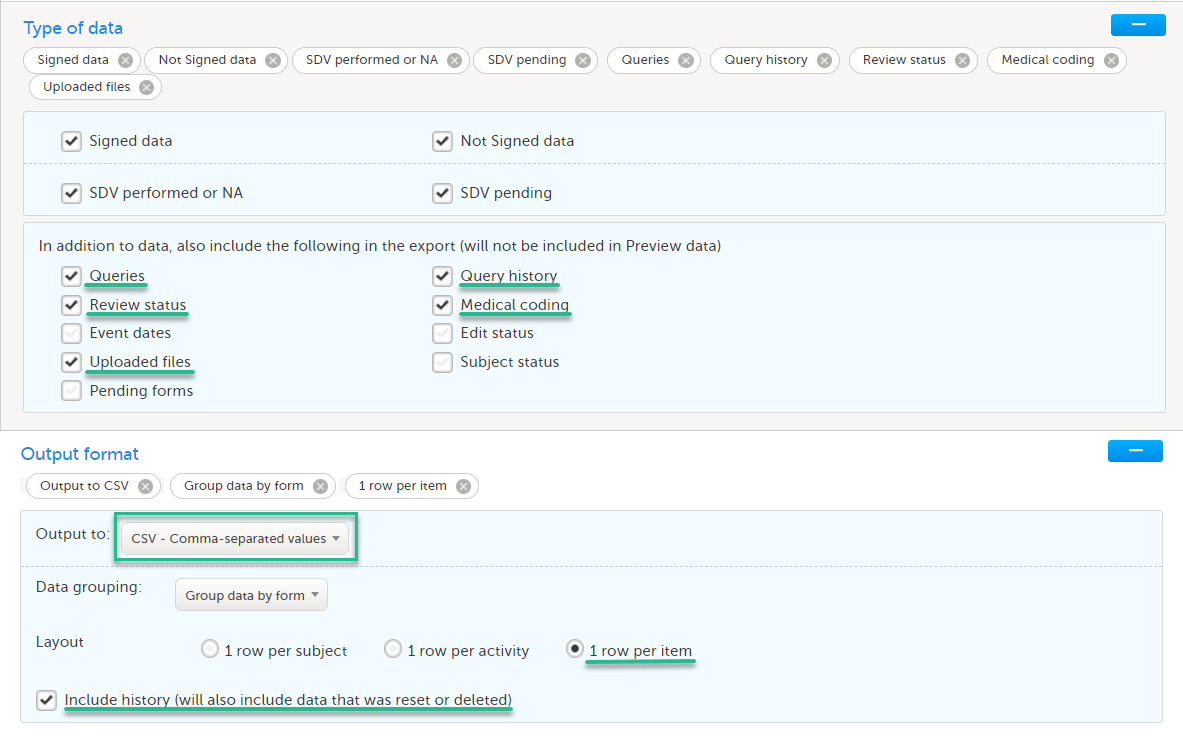
- CSV
- 1 row per item
- Include history (will also include data that was reset or deleted)
- Queries, Query history, Review status, Medical coding, Uploaded files
- CSV
- Include corresponding SAS script
- Queries, Query history, Review status, Medical coding, Uploaded files

- ODM
- Queries, Medical coding, Review status

- Queries, Medical coding, Review status
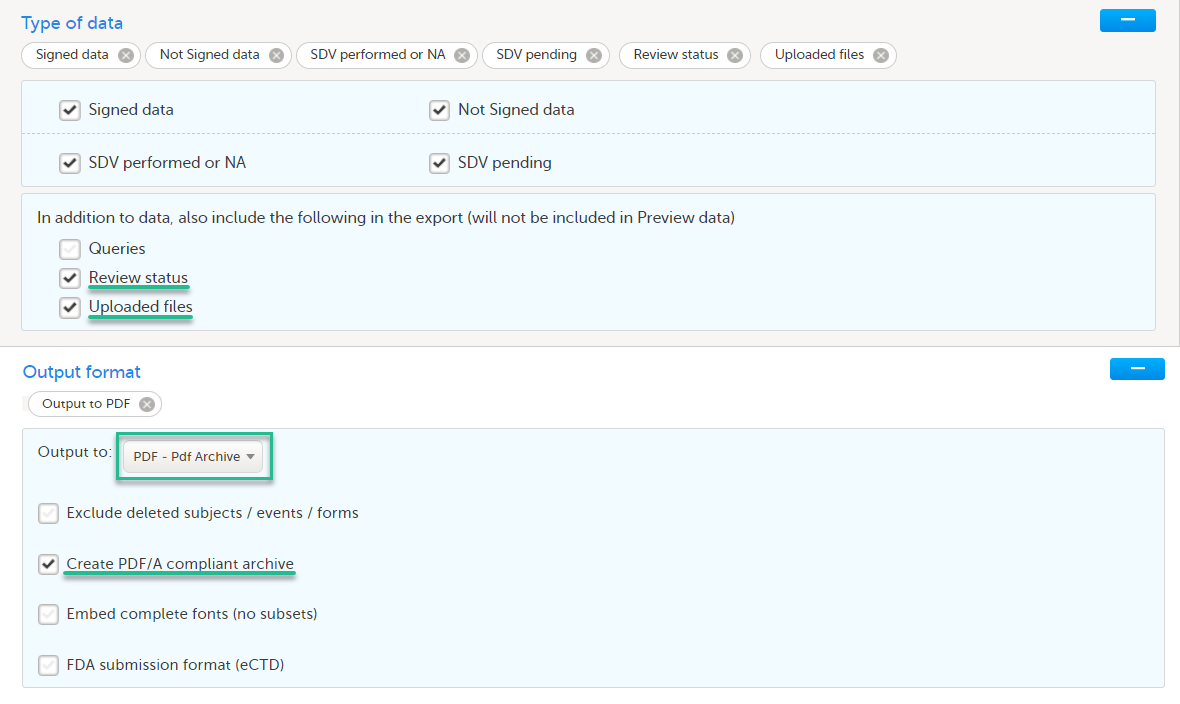
- PDF
- Create PDF/A compliant archive
- Review status, Uploaded files
- Excel
The data export in Viedoc supports all file formats that are required for archiving and regulatory purposes, including these formats:
- Portable Document Format Archive (PDF/A) - an International Organization for Standardization (ISO)-standardized version of the PDF format, specialized for archiving and long-term preservation of electronic documents.
- Office Open Extensible Markup Language (XML) - a zipped, XML-based file format for spreadsheets (for example Excel), charts, presentations, and word processing documents.
- Statistical Analysis System (SAS) - a format used for statistical analysis in the SAS software suite. A SAS script to import CSV datasets into SAS can be included in the CSV export.
- Operational Data Model (ODM) - a vendor-neutral, platform-independent format for exchanging and archiving clinical research data, along with their associated metadata, administrative data, reference data, and audit information.
