Archiving a study
This is a description of the main steps in the process of archiving a clinical study. The detailed instructions for each step are described in the linked lessons.
Archiving a clinical study is the responsibility of the sponsor (study Trial Master File (TMF)) and the investigators (site TMFs). The study archive should include all study data and metadata, including the study design.
When a study is complete, you typically need to go through the steps below.
Lock study
As a Study Manager, you can lock a study when all events have been completed, reviewed, approved/signed, and no more data will be added to the study.
When a study is locked, it is still possible to view and export data. It is not possible to add or edit any data. It is also not possible to change the study settings or add new sites. However, it is still possible to invite new users to existing sites. These users will then receive read-only access.
For more information, see Locking a study.
Export data
Export study data
You can export study data if your Viedoc Clinic user role is set up with the rights for it. For more information, see the Data export section in Viedoc Clinic User Guide.
Make sure to filter the data export to include the following:
- Audit trail
- Query history
- Medical coding
- Review status
The data export in Viedoc supports all file formats that are required for archiving and regulatory purposes, including these formats:
- Portable Document Format Archive (PDF/A) - an International Organization for Standardization (ISO)-standardized version of the PDF format, specialized for archiving and long-term preservation of electronic documents
- Office Open Extensible Markup Language (XML) - a zipped, XML-based file format for spreadsheets (for example Excel), charts, presentations, and word processing documents
- Statistical Analysis System (SAS) - a format used for statistical analysis in the SAS software suite
- Operational Data Model (ODM) - a vendor-neutral, platform-independent format for exchanging and archiving clinical research data, along with their associated metadata, administrative data, reference data, and audit information
Export study design
As part of the TMF structure, the sponsor should define the exact details of:
- What data should be exported from Viedoc for archiving
- When data should be exported
- How data will be archived
You can export the study design from Viedoc Designer. For more information, see Exporting/Locking/Deleting a study design. If a study has more than one version of the study design, remember to export all versions.
Download user logs
On the Users page, select to group the users by Studies.
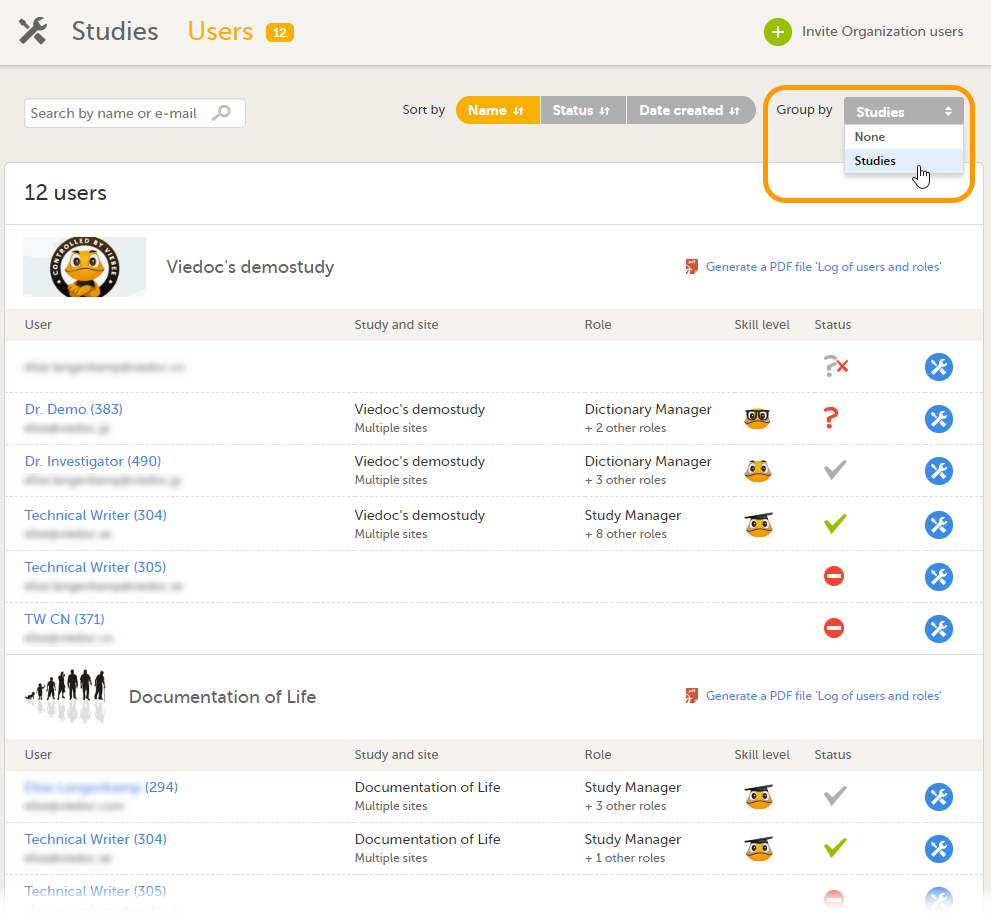
Scroll to the study from which you would like to download the user log and select User logs to open the dropdown menu.
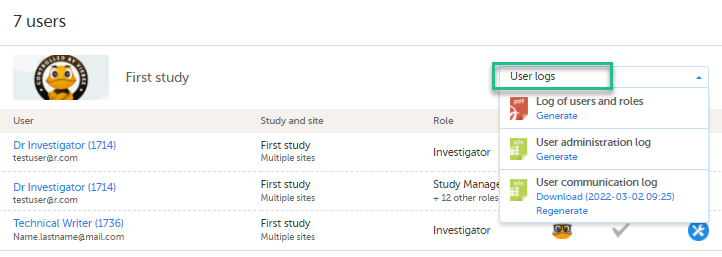
If the log was not previously generated, click Generate a PDF file or Generate an Excel file. If the log was previously generated, the most recent version is stored on the server, shown with a date and time stamp. You can download it by selecting the link, or generate an updated version by clicking Regenerate.
Note! The user logs are generated in the language set by the user who is generating the log. Therefore, the previously generated file is available for download only if it was generated in the language you have currently set in Viedoc.
Delete a study
A study can be permanently deleted from Viedoc when the study is locked. Deletion is initiated by the Study Manager, who can submit a request to delete the study to the Organization Administrator. The Organization Administrator can then approve or reject the request.
After study deletion is approved by the Organization Administrator, the study is shelved in Viedoc's database. A deleted study is not visible in Viedoc Clinic or Viedoc Designer, the study is only displayed in the study overview page of the Organization Administrator in Viedoc Admin.
The Organization Administrator can revert the deletion of a study within 180 days after the deletion request has been approved. After this period, the study, including all study details and data, will be permanently deleted from the Viedoc database. It will not be possible to find any traces of the study and the subjects included.
For more information, see:
