Configuring user roles
Configuring user roles
The Roles page
Roles are configured on the Roles page. In Viedoc Designer, on the Overview of study design page, select Edit in the Roles field to open the Roles page.
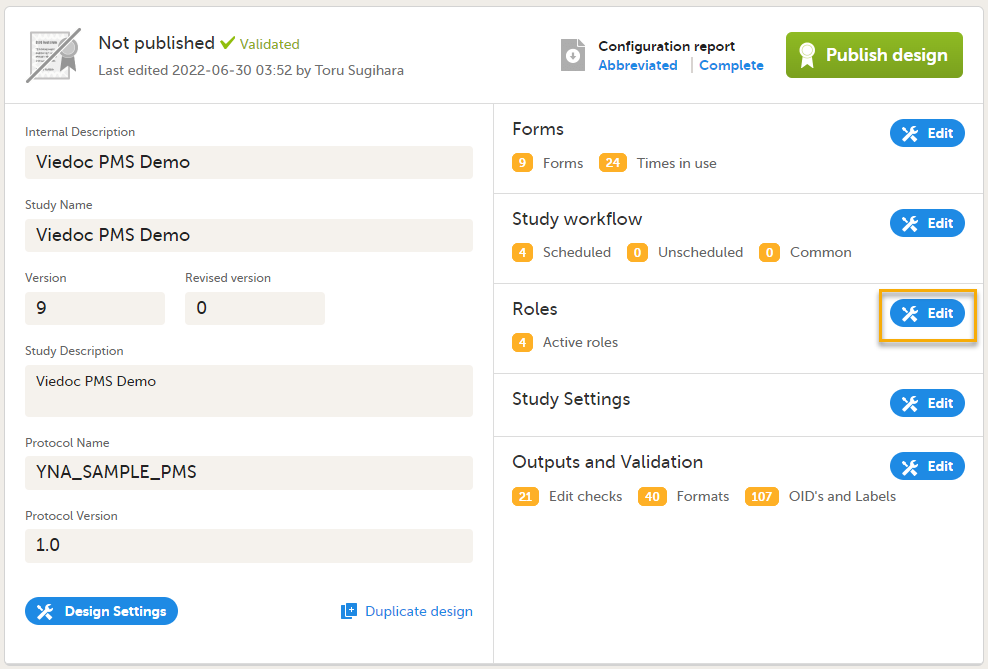
Configuring roles
The Roles page allows you to edit, view, add, copy, and delete user roles. Select the Pen icon to open the Edit roles menu and set the rights for each role.
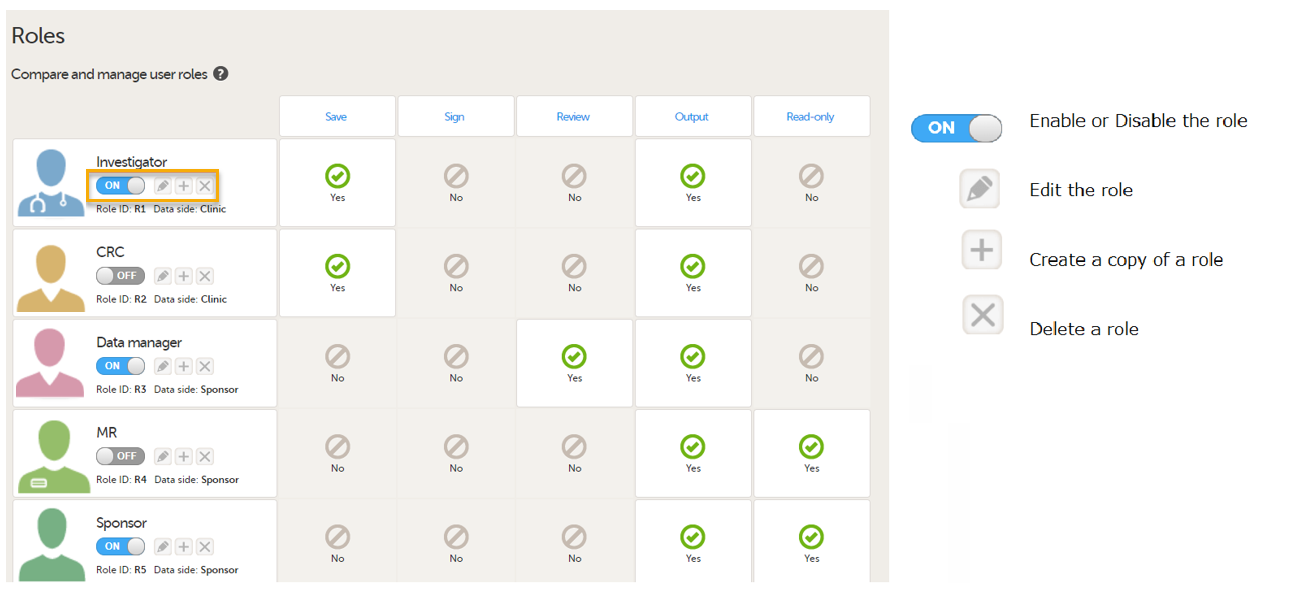
For PMS studies, there are Clinic side and Sponsor side Edit role pages where you can choose to edit roles for either the Clinic Side or the Sponsor side. For more information about how to edit these pages, see Configuring roles.
Clinic side Edit role page
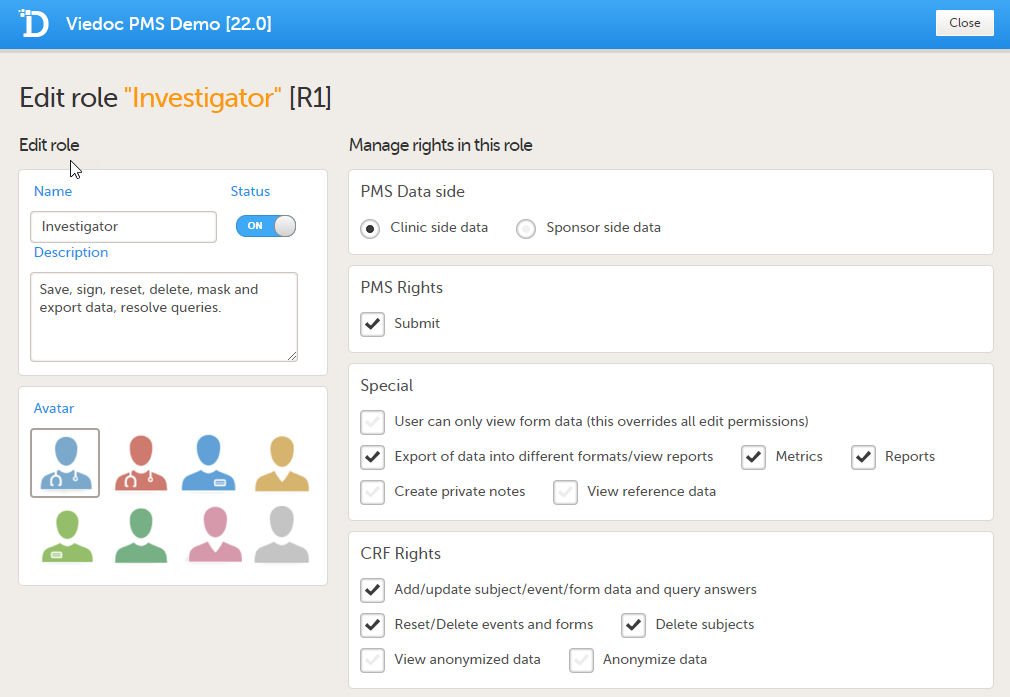
Sponsor side Edit role page
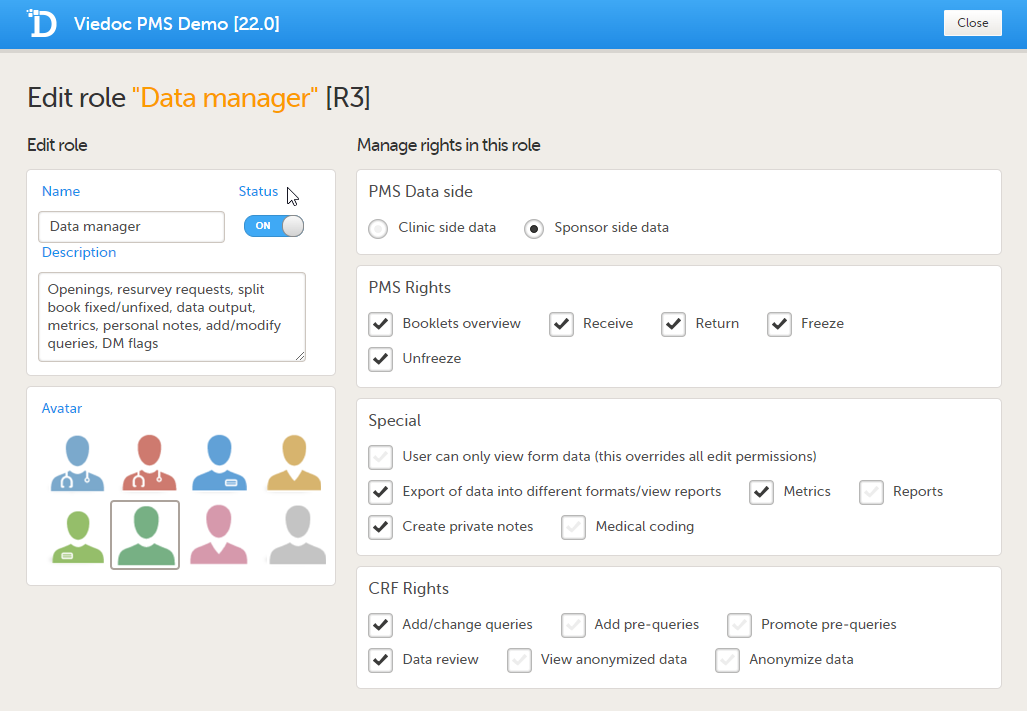
User rights
The following rights can be selected:
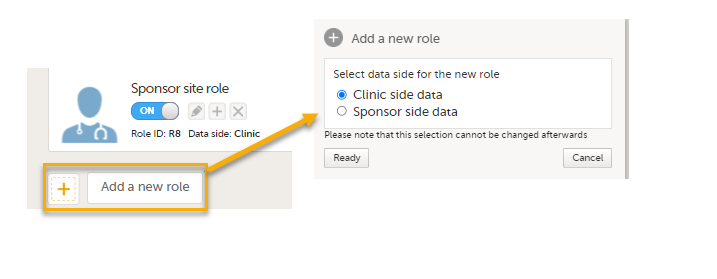
PMS Data side
- Clinic side data
- Sponsor side data
Note! You can only select a data side when you add a new role. The PMS Data side can NOT be changed after a role has been added.
PMS rights - booklet permissions
Clinic Side:
- Submit: User can submit booklets
Sponsor side:
- Booklets Overview: User can see Booklets overview on the study start page
- Receive: User can receive booklets
- Return: User can return booklets
- Freeze: User can freeze booklets
- Unfreeze: User can unfreeze booklets
Special rights - rights that give access to specific features
- User can only view form data (this overrides all edit permissions)
- Export of data into different formats/view reports
- Metrics
- Reports (only visible when Metrics is selected). For export/download rights in Viedoc Reports, the user must also have "Export of data into different formats/view reports" selected. The rights may not be applied directly due to the 24-hour data sync in Viedoc Reports.
Note!
For Demo sites to work with Viedoc Reports, a user must be invited with direct site access (not through All sites site group).
For Production sites, Viedoc Reports is available for all site groups (All sites and the country-specific groups).
- Create private notes
- Medical coding, and if selected (Sponsor side):
- Perform medical coding
- Approve medical coding
- View reference data, and if selected (Clinic side):
- Edit reference data
- Publish reference data
CRF rights - rights regarding adding/editing/saving data and queries
Clinic Side:
- Add/update subject/event/form data and query answers
- Reset/Delete events and forms
- Delete subjects
- View anonymized data
- Anonymize data
Sponsor Side:
- Add/Change queries
- Add pre-queries
- Promote pre-queries
- Data review
- View anonymized data
- Anonymize data
Using predefined roles
By default, a set of predefined roles is set up by the system, and it can be modified for your study. The default roles and default permissions for the Clinic side and the Sponsor side are listed in the following tables:
| Role | PMS rights | Special rights | CRF rights |
|---|---|---|---|
| Investigator (Clinic side) | Submit booklets | - Export of data into different formats/view reports |
- Add/update subject/event/form data and query answers |
| CRC (Clinic side) | Export of data into different formats/view report | - Add/update subject/event/form data and query answers -Reset/Delete events and forms - Delete subjects |
|
| Data Manager (Sponsor side) | - Booklets overview - Receive booklots - Return booklets - Freeze booklets - Unfreeze booklets |
- Export of data into different formats/view reports - Metrics - Reports - Create private notes |
- Add/change queries - Data review |
| MR (Sponsor side) | - The user can only view form data (this overrides all edit permissions) - Export of data into different formats/view reports - Metrics - Reports |
||
| Sponsor (Sponsor side) | - The user can only view form data (this overrides all edit permissions) - Export of data into different formats/view reports - Metrics - Reports |
||
| Data puncher (Clinic side) | Submit booklets | Export of data into different formats/view report | - Add/update subject/event/form data and query answers - Reset/Delete events and forms - Delete subjects |
| Reference Data Editor (Clinic side) | - The user can only view form data (this overrides all edit permissions) - View reference data - Edit reference data - Publish reference data |
||
| Regulatory Inspector (Sponsor side) |
- The user can only view form data (this overrides all edit permissions) |
- View anonymized data |
