Overview of Viedoc
Introduction
Viedoc is a service over the internet system for managing Case Report Form (CRF) data in clinical studies and patient registries.
Viedoc is an Electronic Data Capture (EDC) system that enables easy data capture, management, validation and presentation of clinical trial data. Viedoc is a Software-as-a-Service (SaaS) accessed directly through a web browser and requires no installation. It is intuitive and user-friendly and enables efficient sharing of information.
Viedoc is a study centric system, that is, all the functionalities are more or less related to a specific study. Usually a study in Viedoc corresponds to a clinical trial or other types of projects where data collection is applicable.
The main functionalities provided by Viedoc are:
- Data handling:
- Subject screening
- Online data entry (eSource compliant)
- Automatic data transfer
- Data signing
- Commenting
- Medical coding
- File uploads
- Randomization and Trial Supply Management (RTSM)
- Randomization and advanced allocation
- Viedoc Logistics
- Output:
- Export to the following formats:
- Automatic creation of empty and annotated CRFs
- Audit trail
- Data Quality Metrics
- Study statistics
- Data review/Monitoring:
- Source-Data Verification (SDV)
- Clinical/Data Review & Lock
- Pre-query & Query Handling
- Other:
- Single point of login
- 24/7 technical support
The following diagram is an overview of the main Viedoc interactions and functionalities:
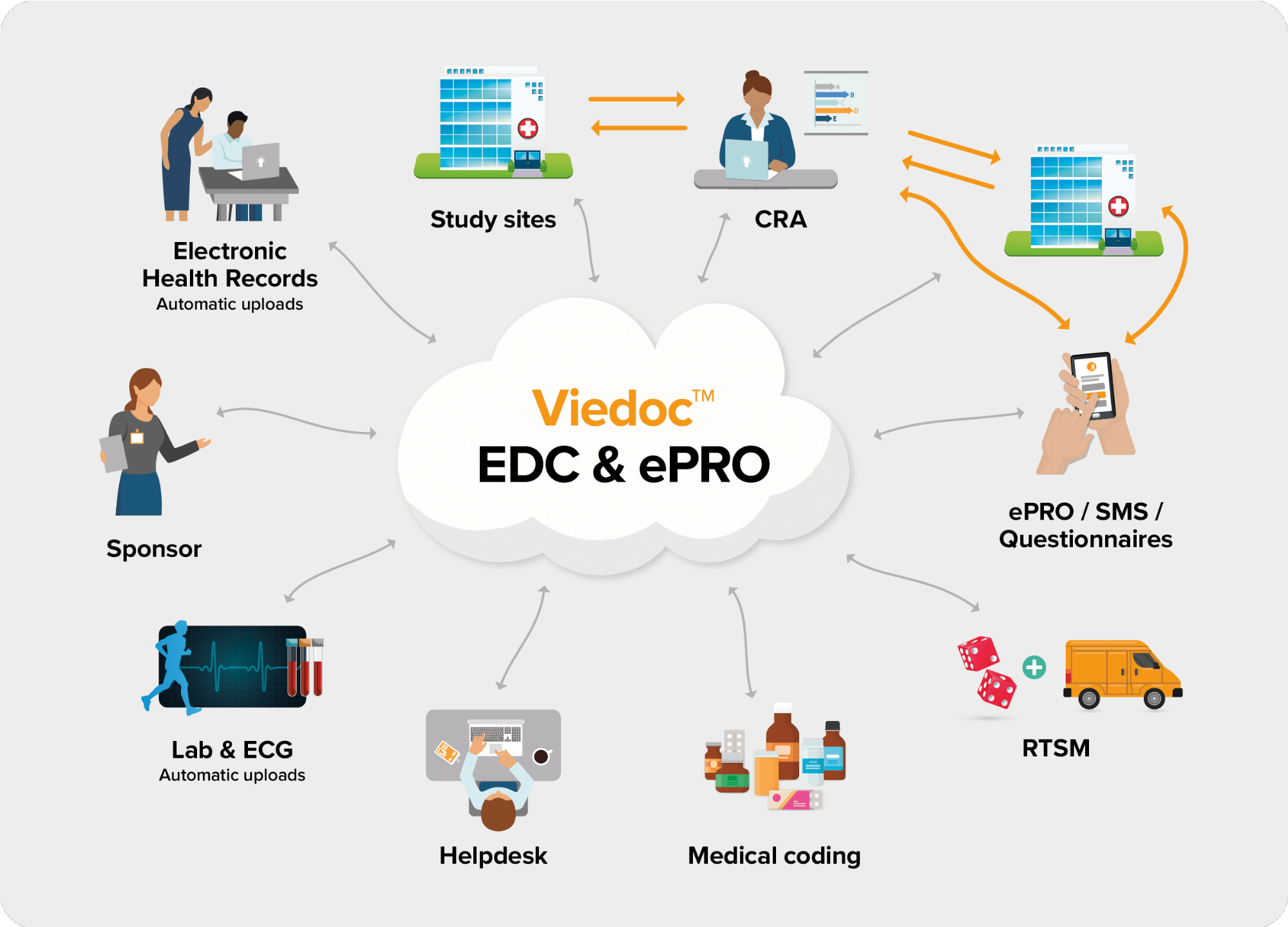
Viedoc is compliant with all relevant guidelines, standards and regulations in Europe, North America and Japan, including:
- Food and Drug Administration (FDA) 21 Code of Federal Regulations (CFR) part 11
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) - Good Clinical Practice (GCP)
- Clinical Data Interchange Standards Consortium (CDISC)
- Computerized Systems Used In Clinical Investigations (CSUCI)
- Health Insurance Portability and Accountability Act (HIPAA)
- Developed according to Good Automated Manufacturing Practice (GAMP) 5
- General Data Protection Regulation (GDPR)
A study in Viedoc
Study sites
Every study has at least one study site, which corresponds to a clinic. A Viedoc user can have access to one or several studies in Viedoc and for one study the user can have access to one, several or all study sites. A Viedoc user is linked to a study site using a user role. A single user can have one or several roles for a study site and can also have different roles for different sites.
Events and forms
During a study, there are typically a number of questions to be answered and completed with data about the subject. A group of questions that belong together are captured in a form. Forms can be event-dependent or event-independent (log forms / common events). Event-dependent forms are linked to a specific event and the data belonging to these forms is registered during or in relation to a study event. Event-independent forms can be used to report data or events that happen before, between, or after events. Medical history events, concomitant medications, or adverse events are examples of forms that can be captured in event-independent forms.
Subjects
All study subjects are identified using a unique subject key. In addition to the subject key, a subject can be identified using background information such as gender, initials, or date of birth. The subject’s background information is usually entered when adding the subject in the system and will most likely not change during the course of a study.
System architecture
The Viedoc platform
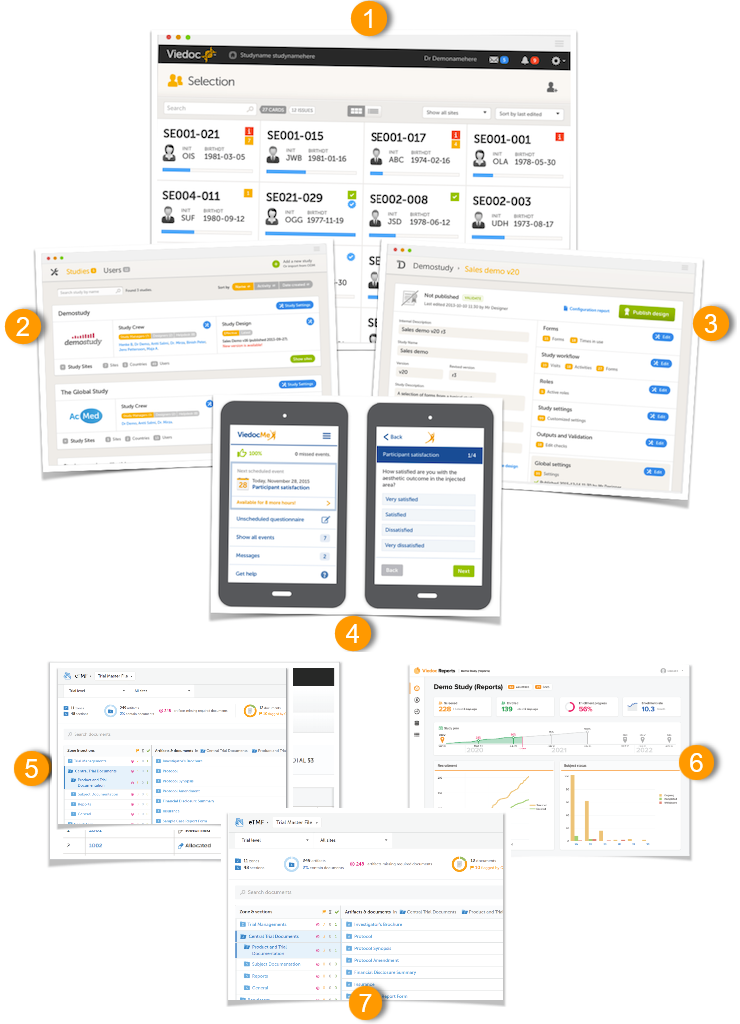
The Viedoc platform consists of seven different applications:
- Viedoc Clinic - for site staff and project team members that need to have access to CRF data.
- Viedoc Admin - for parts of the study team to handle users, sites and updates in study designs. Viedoc Admin is the interface where you initiate a new study, manage the study while it is running and finally close the study.
- Viedoc Designer - for the study developer to design the study. Viedoc Designer is the tech part where you design forms, set the study workflow, add roles, prepare the randomization, add edit checks and much more.
- Viedoc Me - the subject diary, or electronic Patient Reported Outcome (ePRO). All subject questionnaires are easily completed and submitted by the subject through this application.
- Viedoc Logistics - for supply managers who handle the Investigational Products (IPs) of your study.
- Viedoc Reports - for viewing and analyzing study progress and performance.
- Viedoc eTMF - for capturing, managing, sharing, and storing essential documents for your study in a digital repository.
System languages
Viedoc Clinic and Viedoc Logistics are available in the following languages:
- Chinese (Simplified)
- Chinese (Traditional)
- English
- French
- German
- Japanese
- Polish (not available in Logistics)
- Portuguese
- Spanish
- Swedish
Viedoc Admin and Viedoc Designer are available in the following languages:
- Chinese (Simplified)
- English
- German
- Japanese
- Swedish
Viedoc Me is available in the following languages:
- Afrikaans
- Arabic
- Bulgarian
- Chinese (Simplified)
- Chinese (Traditional, Hong Kong S.A.R.)
- Chinese (Traditional, Taiwan)
- Chinese (Traditional)
- Croatian
- Czech
- Danish
- Dutch
- English
- Estonian
- Finnish
- French
- French (Belgium)
- Georgian
- German
- Greek
- Hebrew
- Hebrew(Israel)
- Hungarian
- Italian
- Japanese
- Kazakh
- Korean
- Latvian
- Lithuanian
- Malay
- Norwegian (Bokmål)
- Norwegian (Nynorsk)
- Polish
- Portuguese
- Romanian
- Russian
- Serbian (Cyrillic)
- Serbian (Latin)
- Setswana
- Slovak
- Slovenian
- Southern Sotho
- Spanish
- Swedish
- Thai
- Turkish
- Ukrainian
- Vietnamese
- Xhosa
- Zulu
Viedoc Reports is available in the following languages:
- Chinese (Simplified)
- Chinese (Traditional)
- English
- Japanese
- Swedish
Viedoc TMF is available in the following languages:
- Chinese (Simplified)
- English
- Japanese
- Swedish
To change the language, see Manage your Viedoc account.
If you require any additional language that is not listed above, please contact your Viedoc representative.
Note! Viedoc does not allow users to use a default browser translation within the system. This prevents individual users from overriding the chosen system language and agreed upon terminology and formulations.
eLearning
The following table shows the current eLearning curriculums and the language versions.
The green curriculums are the main user guides for the different applications.
The orange curriculums are role-specific ones, meaning they are tailor-made for our different users.
| Curriculum | English | Chinese | Japanese | URL |
|---|---|---|---|---|
| Viedoc Clinic User Guide | x | x | x | https://help.viedoc.net/c/47e0ad |
| Viedoc User Guide for Monitors | x | x | x | https://help.viedoc.net/c/c63e06 |
| Viedoc User Guide for Site Users | x | x | x | https://help.viedoc.net/c/94d6f0 |
| Viedoc User Guide for Data Managers | x | x | x | https://help.viedoc.net/c/1994d8 |
| Viedoc User Guide for Project Managers | x | x | x | https://help.viedoc.net/c/04361f |
| Viedoc User Guide for Medical Coders | x | x | x | https://help.viedoc.net/c/3108de |
| Viedoc Admin User Guide | x | x | x | https://help.viedoc.net/c/331b7a |
| Viedoc Designer User Guide | x | x | x | https://help.viedoc.net/c/e311e6 |
| Viedoc Logistics User Guide | x | x | x | https://help.viedoc.net/c/4a40d5 |
| Viedoc Reports User Guide | x | x | x | https://help.viedoc.net/c/8a3600 |
| Viedoc eTMF User Guide | x | x | https://help.viedoc.net/c/88fc29 | |
| Viedoc User Guide for eTMF Managers | x | x | https://help.viedoc.net/c/fd74dc | |
| Viedoc PMS User Guide for Clinic Side Users | x | x | https://help.viedoc.net/c/91715f | |
| Viedoc PMS User Guide for Sponsor Side Users | x | x | https://help.viedoc.net/c/590df1 | |
| Viedoc PMS Designer User Guide | x | https://help.viedoc.net/c/ed5d47 | ||
| Viedoc User Account Management Guide | x | https://help.viedoc.net/c/508fda |
Organizations
Studies are grouped in Viedoc under organization(s); that is, each client has its own organization where all studies belonging to that organization are stored. By default, one organization administrator is appointed to each organization. This person has been trained by a Viedoc Product Specialist and is responsible for providing access to users within the organization and for adding new studies to the platform.
| Important! It is the responsibility of the organization administrator to make sure that all users within the organization have received appropriate training for their respective tasks. |
System environment
As a Viedoc client, you will be provided with access to two separate environments/instances: one for test/development studies and one for production studies. The purpose of the test/development environment is to allow the evaluation and use of Viedoc without the need of a contract for a specific ongoing study.
Any study that is to be taken in production is normally initiated on the test/development environment and later moved to the production environment once it is “ready” to be shared with the Sponsor or other external party. Please observe that a study in the production environment can be set to operate in demo mode by adding a site of the type “training” to it.
Note! The demo mode of a production study should not be confused with a study in the test/development environment. The purpose of the demo mode is to allow site staff access to specific training site(s) in order to gain sufficient knowledge of the system before accessing production data. When a study has sites with both production and training types added, a switch will be available in Viedoc Clinic. This offers a choice of which mode the data will be entered to - demo or production.
Studies and study designs can be easily transferred from one environment to the other via the ODM export and import feature.
Contact your organization administrator to get access to the respective area.
Note! There is no guarantee that studies running on the test/development environment are completely and continuously backed-up. This environment should therefore never be used for any production studies.
Licensing
All production studies need to have a valid license before they can be taken into production. The license is provided by a Viedoc representative. The license fee for the study is based on several factors such as duration, number of sites and patients, among others. The license fee is charged starting with the first patient added and for the duration of the study; which means, until the study is locked in Viedoc. If the study is not deleted from the database within 2 months, a post-study access fee may apply.
Every license is connected to a reference ID. The reference ID can be found on the signed study work order and should be entered in the field Reference ID in the Study settings in Viedoc Admin (nr. 1 in the image):
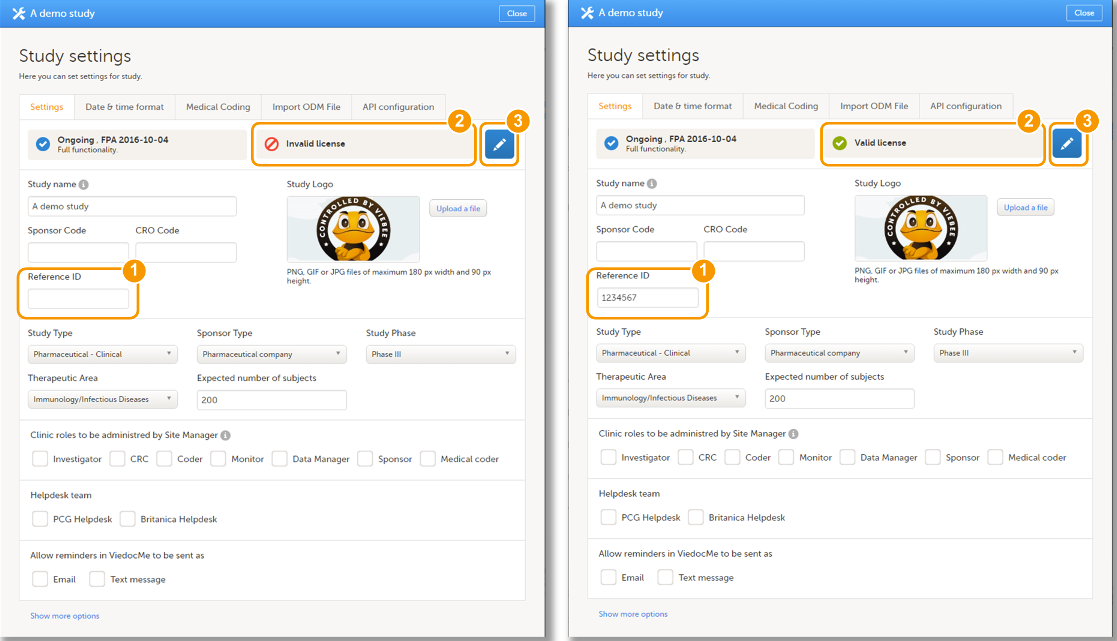
Upon entry of the reference ID, the reference ID is verified. If the reference ID is valid, the text Valid license key will be indicated at the following places:
- Study settings in Viedoc Admin (nr. 2 in the image)
- Studies list in Viedoc Admin
- Study status in Viedoc Admin (nr. 3 in the image)
Once the reference ID has been verified, the study can be taken into production. A study is in the production mode once Production is selected as a site type. As soon as at least one site of production type is added, the Reference ID is locked and there is no way to unlock it afterwards.
For more information regarding license fee and reference ID, please contact your Viedoc representative.
Keep yourself updated!
Viedoc is being developed at a rapid pace. To make sure you are using the platform correctly and to its full potential, use this guide as a refresher after every new release.
Brief information about new and updated functionality after every release can be found in:
- the Release notes, which are sent out before every release, and can be downloaded from the Viedoc website, click:
- the eLearning, in What's new in the latest release?
