Overview of the submit-receive-return function
Introduction
Viedoc PMS offers support for collecting data in booklets, allowing data to be collected during a specific time period rather than a specific event date, and support for sending and receiving booklets back and forth between site and sponsor. This process of handling booklets in a Japanese post-marketing surveillance study is known as the Kaifu process. In the Kaifu process, the clinic user chooses when to share data with the sponsor and the sponsor-side user chooses when to receive the data. One of the important characteristics of this process is that the sponsor-side user does not have access to any data entered in a booklet, until the booklet has been submitted by the clinic, and a receive action has been actively performed by the user at the sponsor side.
Booklets can only be submitted to the sponsor when all events and forms in the booklet are completed.
There is an exception to this rule, which depends on your study configuration. It is possible that some forms, (for example, but not limited to, Adverse Events) can be managed, that is, submitted/received/returned/reviewed/reported individually. When these forms are saved, a warning message is displayed and a Manage link is available. For more information, see Submitting and recalling booklets, AE reports and forms and Handling booklets, AE reports and forms for more information.
This lesson provides a description and a schematic overview of the submit-receive-return process of handling booklets and adverse events.
Handling booklets
Schematic overview of the Kaifu process
The below image provides a schematic overview of the submit-receive-return process of handling booklets.
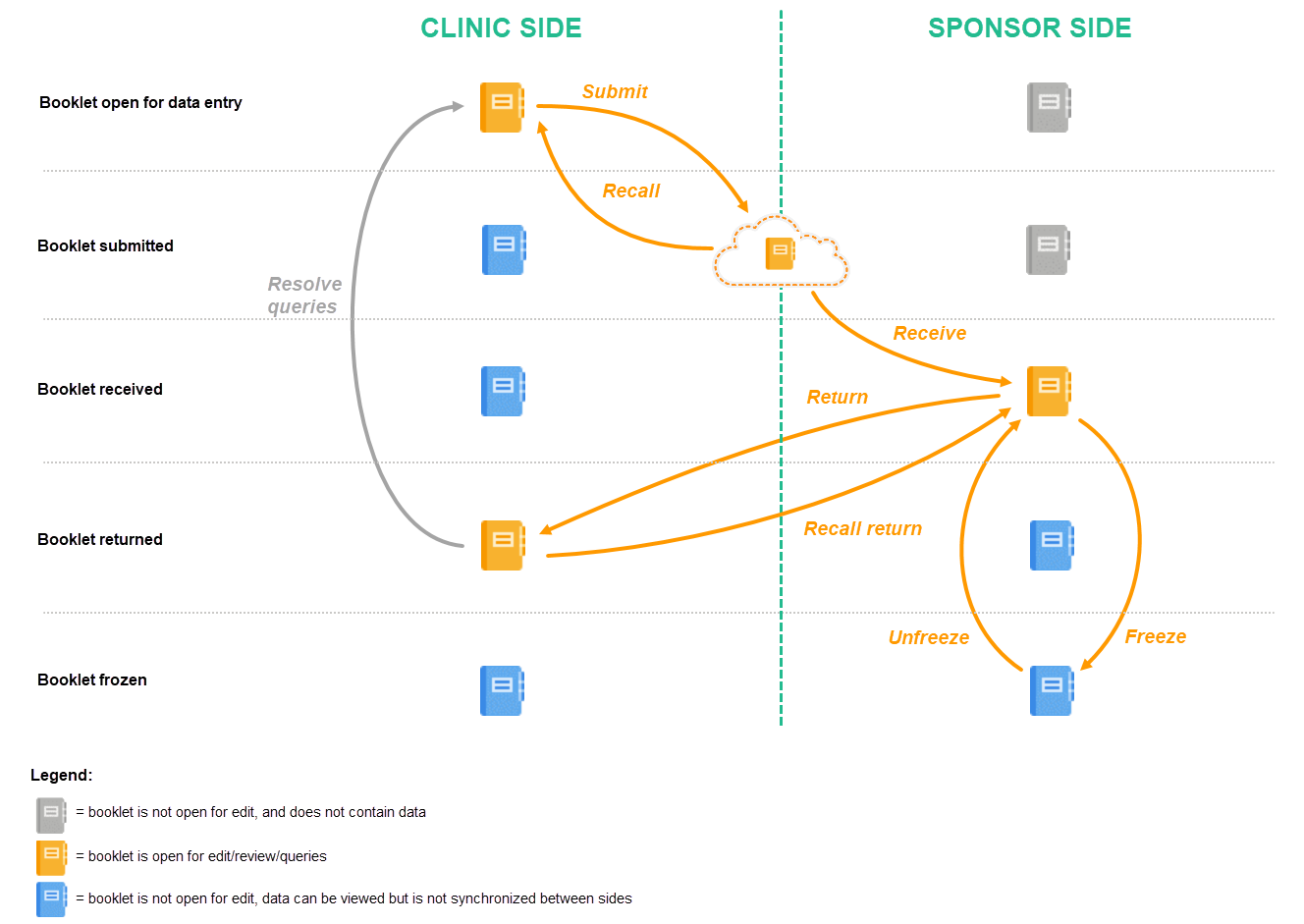
Description of the workflow during the Kaifu process
A booklet, a compilation of data being collected during a specific period of time, is initiated on the clinic side and data are entered. Once a booklet is completed and all required items are filled in, the booklet can be submitted. The clinic user has to confirm submission of the booklet by entering his/her password. By giving the password, the user at the same time signs the data in the booklet. Submission of a booklet does not immediately result in the booklet appearing at the sponsor side, instead, the sponsor side user has to actively receive the submitted booklets. A submitted booklet can be recalled at any time up until the moment that the sponsor side has received the booklet. A recalled booklet can then be edited before submitting it again to the sponsor side.
On the sponsor side, the booklet receive action has to be confirmed by clicking Confirm. Only once a booklet is received at the sponsor side, is the data in the booklet displayed to the user at the sponsor side. The sponsor side user can review the data and raise queries, while at the clinic, the booklet is in read-only state and no data changes can be made. The sponsor user has the possibility to either return the booklet to the clinic, in case queries are raised for example, or freeze the booklet if all data are reviewed, all possible queries are closed, and the booklet is ready to be shelved. It is possible for the sponsor side user to unfreeze a booklet that has been frozen. This opens up the booklet for raising queries and returning it to the clinic.
All submit, recall, receive, and return actions are recorded in the audit trail.
The actions during the Kaifu process
The actions in the submit-receive-return process are summarized in the table below.
| Action | Executed on which side? | When? | State of the data in the booklet | |
|---|---|---|---|---|
| Clinic side | Sponsor side | |||
| Initiate booklet/data entry | Clinic side | When adding a subject, and when the time period for a booklet has passed. | Open for data edit | No data can be viewed* |
| Submit to sponsor side |
Clinic side | When all required items are filled in. | Read-only | No data can be viewed until Receive. |
| Recall to clinic side |
Clinic side | When the booklet is submitted, but not yet received on the sponsor side* | Open for data edit | No data can be viewed* |
| Receive | Sponsor side | When the booklet is submitted, and not recalled to the clinic side. | Read-only | Read-only, but open for data review and raising queries |
| Return to clinic side |
Sponsor side | When the booklet is received. | Open for data edit** | Read-only, no actions |
|
Recall return |
Sponsor side | When the booklet is returned but not handled by the clinic side. | Read-only | Read-only, but open for data review and raising queries |
| Freeze | Sponsor side | When the booklet is received, all data are reviewed and all queries are closed. | Read-only | Read-only, no actions |
| Unfreeze | Sponsor side | When the booklet is frozen. | Read-only | Read-only, but open for data review and raising queries |
*The subject is not displayed at the sponsor side until the first booklet of this patient has been submitted and received by a sponsor side user. From that moment on, the submitted booklet with its data, and the structure of the remaining booklets (yet without data) are displayed at the sponsor side. The events and forms inside any not-submitted booklet can be viewed, but the forms cannot be opened.
**The booklet needs to be submitted by the clinic side user again to continue in the workflow.
Handling adverse events
AE reports
AE forms can be submitted, recalled, received, and returned in two ways:
- As part of a complete booklet submission/recall/receive/return.
- As an individual AE report.
When an AE is registered before the booklet is ready to be submitted, it is possible to submit the AE separately, that is, without having to submit the entire booklet. Similarly, the sponsor can return only the AE form instead of returning the complete booklet, even if the complete booklet has been submitted and received. A separately submitted or returned AE is called an AE report. AE reports follow the same Kaifu process for submit, recall, receive, and return as booklets.
Similarly to handling booklets, the clinic user has to confirm submission of the AE report by entering his/her password, and by which he/she at the same time signs the data. A submitted AE report can be recalled until the AE report has been received by the sponsor. Only once the AE report is received, the data is displayed at the sponsor side. The sponsor user has the possibility to return the AE report if necessary. All submit, recall, receive, and return actions are recorded in the audit trail.
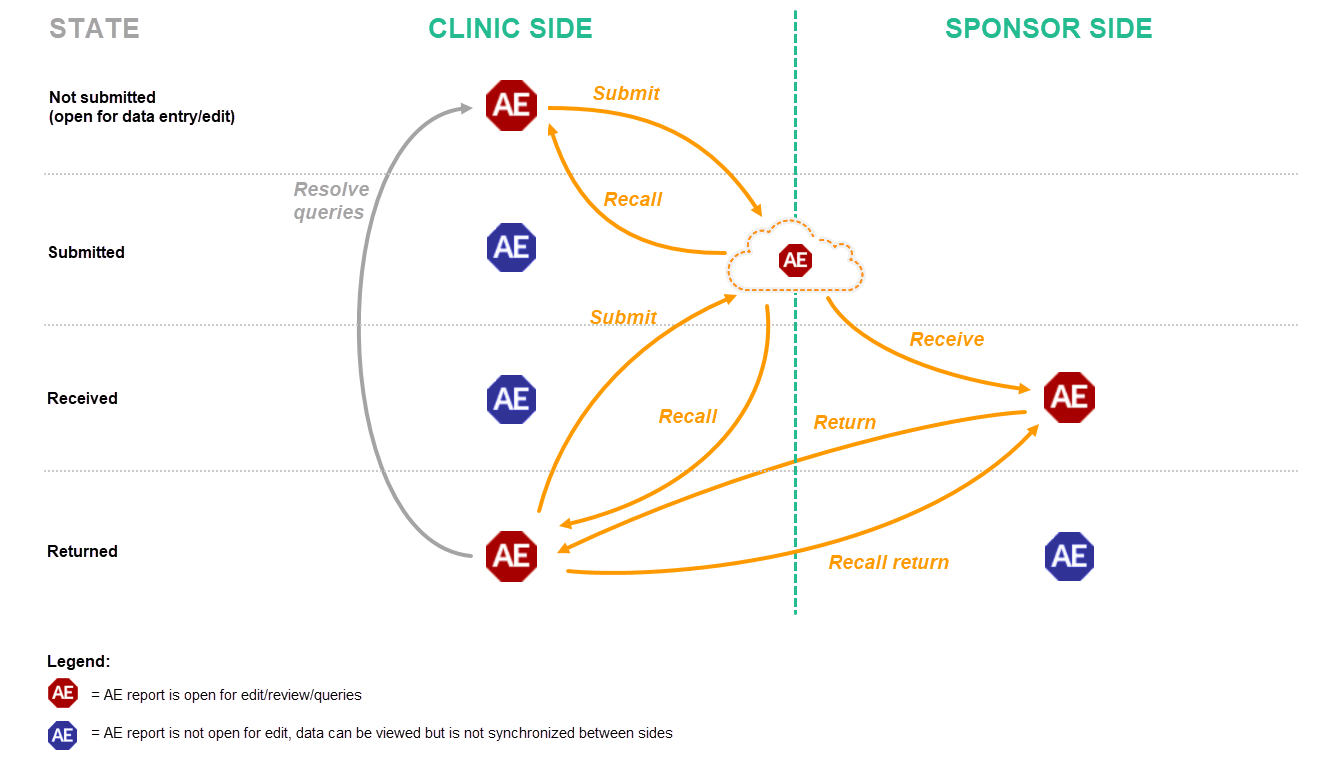
Schematic overview of the submit-receive-return process for adverse events
The following workflows are possible.
- The user on the clinic side submits only the AE report. The user on the sponsor side receives, and if necessary returns, only the AE report. This can be done for example when an adverse event has occurred before the time period of a booklet is finished and the booklet is not ready for submission, or when the user on the sponsor side returns only an AE report and not the complete booklet (as described in workflow 2).
- The user on the clinic side submits the complete booklet. The user on the sponsor side receives the complete booklet, but returns only the AE report. After any possible queries are resolved, the user on the clinic side then has to submit the AE report separately (see the image in workflow 3).
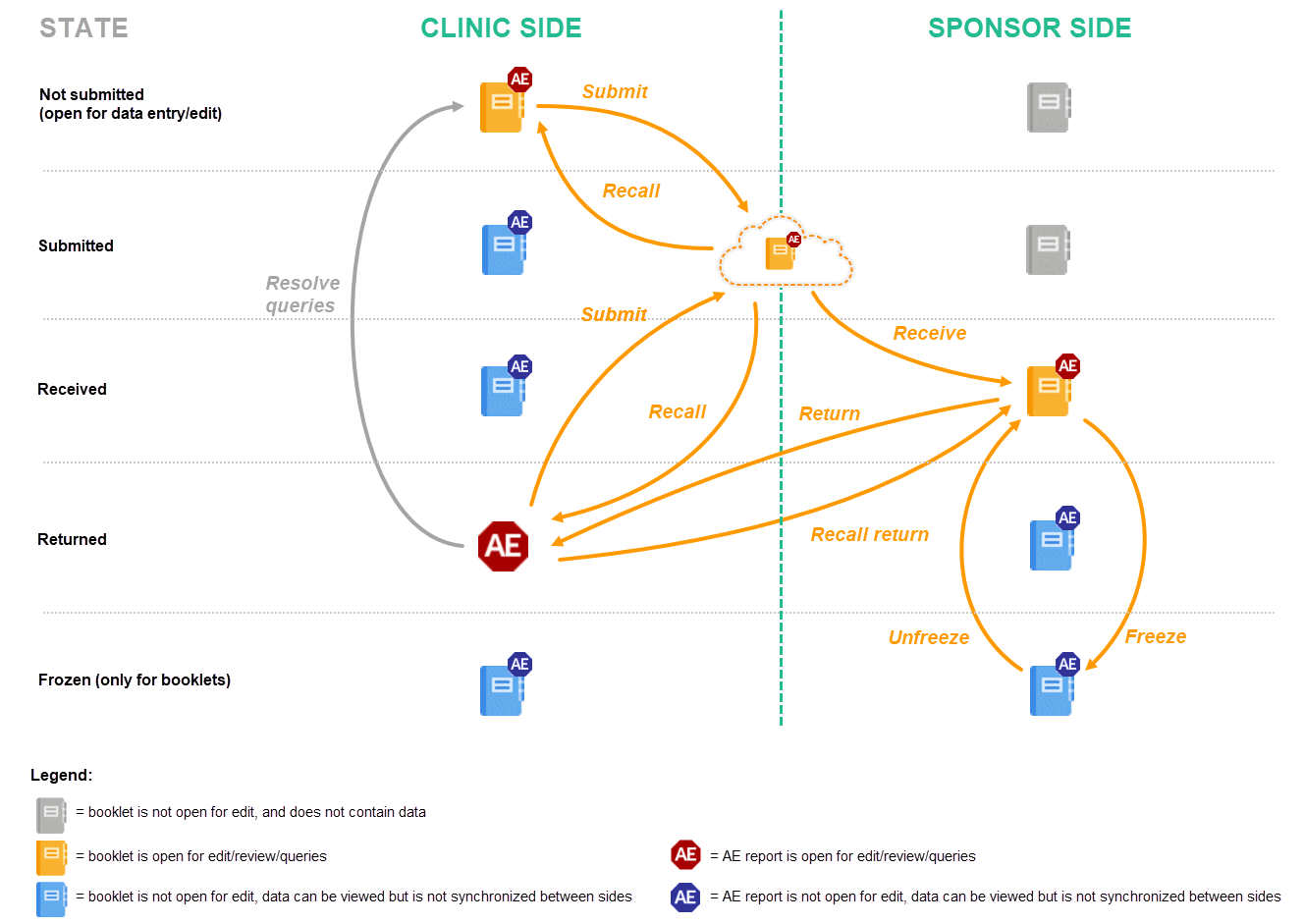
- The user on the clinic side submits the complete booklet, containing the AE. The user on the sponsor side receives the complete booklet, and returns the complete booklet. This workflow is exactly the same as described in Handling booklets.

The actions that can be performed with AE reports are similar to the actions that can be performed with booklets, see The actions during the Kaifu process.
Notes!
- Only booklets can be frozen. It is not possible to separately freeze an AE report.
- If the user on the clinic side submits a booklet, he/she can recall the complete booklet, but cannot recall the AE report only. AE reports can only be recalled if submitted separately.
