Medical coding in Excel export
Introduction
This lesson describes how the medical coding information is structured within an Excel file exported from Viedoc.
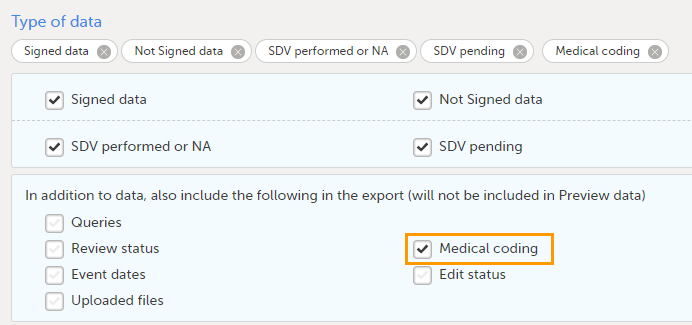
To include the medical coding information in the exported file, you need to select Medical coding under Type of data in the Data Export page:
For general details about data export, see Exporting data.
Medical coding information in Excel
For medical coding, in the Excel export output file, there is one sheet for each dictionary. Only values that have been coded will be present in the export:
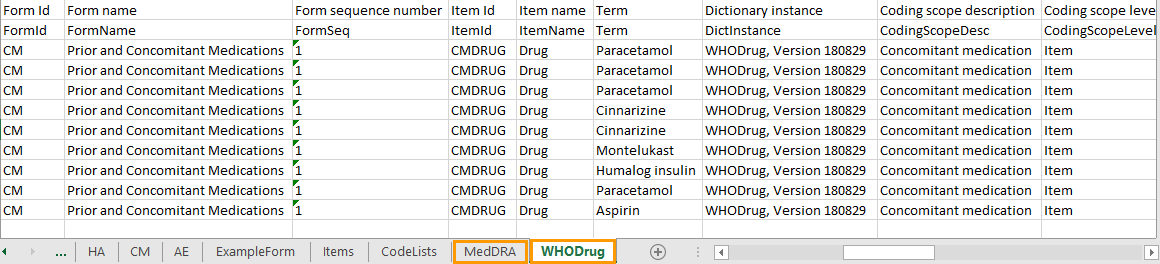
The columns provide information on the item that was coded, followed by the medical coding specific information, as listed in the following table:
| Column name | Description | ||
|---|---|---|---|
| Columns that identify the item | |||
| Site sequence number | A counter that identifies the site globally within the study | ||
| Site name | The site name, as set in Viedoc Admin | ||
| Site code | The site code, as set in Viedoc Admin | ||
| Subject sequence number | A counter that identifies the subject within the site | ||
| Subject Id | The Subject ID, in the format configured in Viedoc Designer. The Subject ID is the subject identifier displayed in Viedoc Clinic on the subject card, subject details page, and so on. | ||
| Event sequence number | A counter that identifies the event within the sequence of events for the same subject | ||
| Event Id | The event ID, as set in the study design (in Viedoc Designer) | ||
| Event name | The event name, as set in the study design (in Viedoc Designer) and displayed in Viedoc Clinic | ||
| Event date | The event date, as set in Viedoc Clinic when the event is initiated | ||
| Activity Id | The activity ID, as set in the study design (in Viedoc Designer) | ||
| Activity name | The activity name, as set in the study design (in Viedoc Designer) and displayed in Viedoc Clinic | ||
| Form Id | The form ID, as set in the study design (in Viedoc Designer) | ||
| Form name | The form name, as set in the study design (in Viedoc Designer) and displayed in Viedoc Clinic | ||
| Form sequence number |
Counter that identifies the instance of the respective form within the respective activity. This is mostly used for repeating forms. For non-repeating forms, this is 1. If a form is reset and then saved again the new form has sequence number 2, and so on. Form sequence number increases one step every time reset/initiate occurs. |
||
| Item Id | The item ID, as set in the study design (in Viedoc Designer) | ||
| Item Name | The item name, as set in the study design (in Viedoc Designer) and displayed in Viedoc Clinic | ||
| Subject form sequence number | Counter that uniquely identifies the instance of a specific form on a subject level, that is, it starts with 1 and it is incremented each time a new instance of the form is created for that subject. | ||
| Origin Subject form sequence number | For a copied form instance, it identifies the form instance from which data was copied for the first time. For the first instance of the form (that is, not copied) it gets the value of the SubjectFormSeqNo. |
||
| Source Subject form sequence number | For a copied form instance, a counter that identifies the source of a copied form instance (the form instance the data was copied from). It gets the value of the SubjectFormSeqNo from which the form instance was copied. For the first instance of the form (not copied) it is empty (null). |
||
| Medical coding-specific information | |||
| Term | The coded term | ||
| Dictionary instance | The description of the dictionary instance, as set in Viedoc Admin when uploading the dictionary | ||
| Coding scope description | The coding scope description, as defined in Viedoc Designer | ||
| Coding scope level | The coding scope level, as defined in Viedoc Designer, can be one of the following:
|
||
| Code sequence number | A counter that identifies the code for those values with more than one code | ||
| Approved by user | The name and ID of the user who approved the items | ||
| Approved on date (UTC) | The date and time (UTC) when the item was approved | ||
| Dictionary-specific information | |||
|
WHODrug:
|
MedDRA:
|
ATC without DDD:
|
IDF:
|
| Interpretation | The medical coder's interpretation of the applied coding value | ||
| Coded by user | The name of the user who performed the coding, followed by the user ID in parentheses | ||
| Coded on date (UTC) | The date and time when the coding was performed | ||
| Version | The dictionary version | ||
| Dictionary-specific information | |||
|
WHODrug:
|
MedDRA:
|
ATC without DDD:
|
IDF:
|
An example - WHODrug in Excel exported file
This section illustrates an example of how the data coded using the World Health Organization Drug Dictionary (WHO DD) dictionary looks in the Excel export output.
There are two different use cases, depending on the which level of granularity is selected when applying the code in Viedoc Clinic:
1. Drug (default)
2. Preferred name
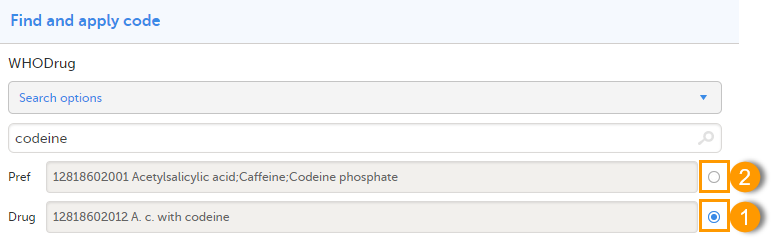
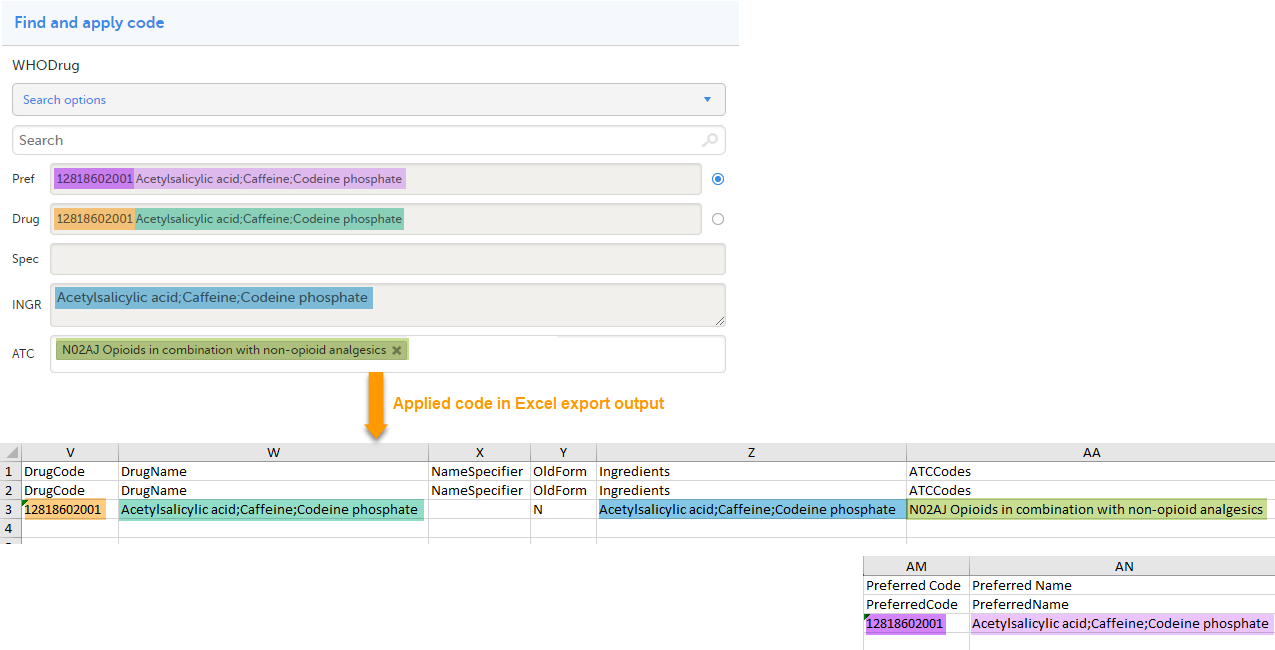
When selecting Drug
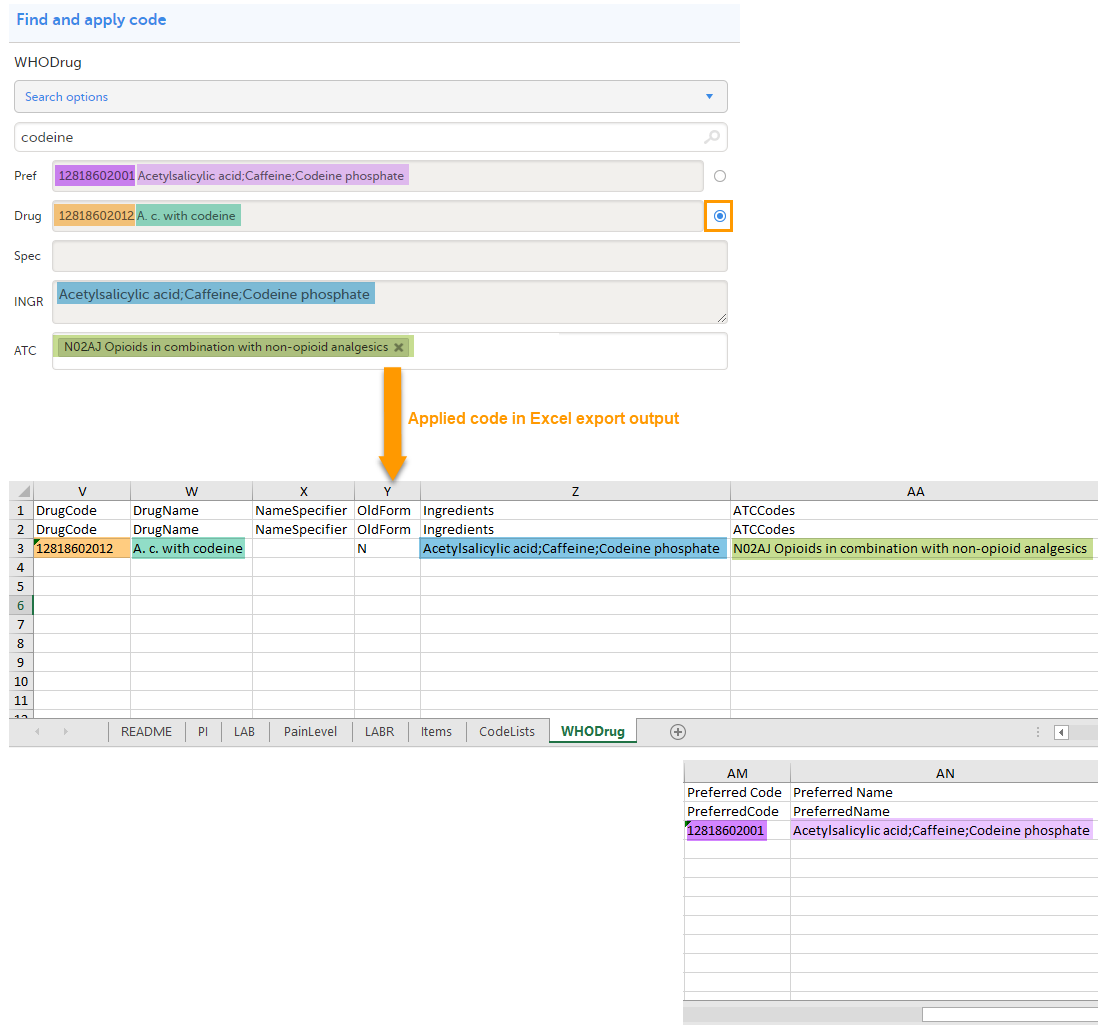
The image below illustrates how the coded data looks in the export output, if the Drug is selected when applying the code in Viedoc Clinic.
When Drug is selected, the Preferred code and Preferred name come out in the export as well, in the last columns, as illustrated in the image.
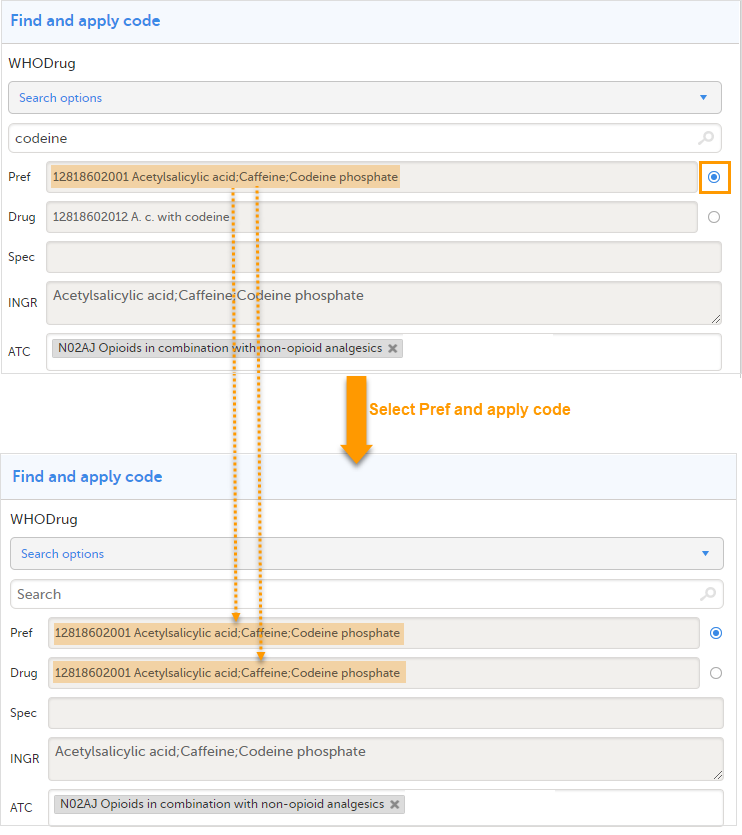
When selecting Preferred name
The image below illustrates how the coded data looks in the export output, if the Preferred name is selected when applying the code in Viedoc Clinic.
After the Preferred name is selected and the code is applied, for the respective coded item, the value of the Preferred name ends up in both Drug and Pref fields, in both Viedoc Clinic, as well as in the exported output.