Adding a new study
Introduction
This lesson provides instructions on how to add a new study. Adding a new study is done in Viedoc Admin. Only the Organization Administrator can add studies.
Note! For all production studies, make sure a contract with Viedoc Technologies exists before proceeding. See Overview of Viedoc for information about licensing.
Adding a study
Note! Adding a new study can only be done by the Organization Administrator.
To add a new study:
| 1 | Open Viedoc Admin and click Show studies in the organization you would like to add a study to. The study overview page opens. | |
| 2 |
Click Add a new study. 
The Add a new study pop-up opens. |
|
| 3 |
Enter a name for the study, and the e-mail address to the person that will be appointed as Study Manager.
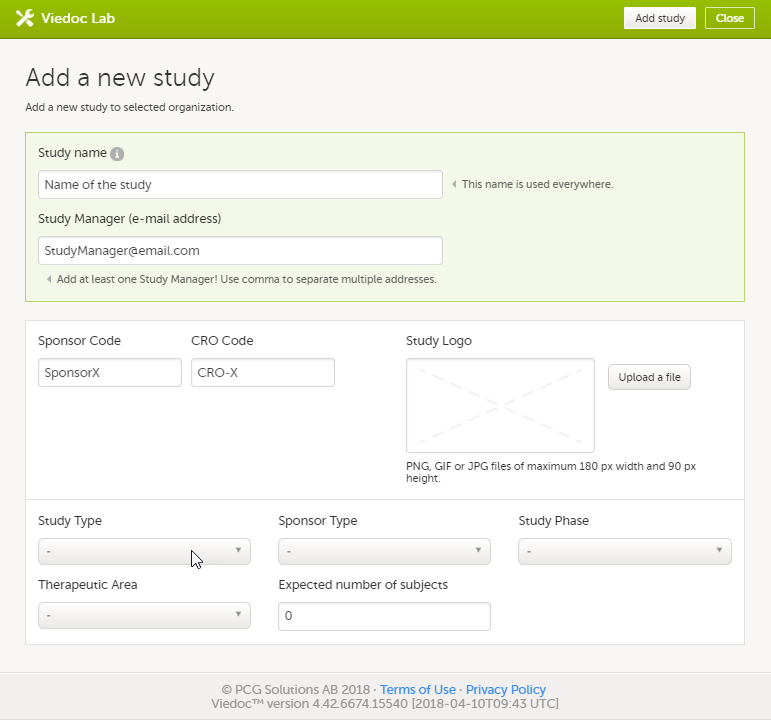
The information in the green area is required. Optionally, you can enter details about the sponsor and the study, but these fields can also be filled in at a later stage by the appointed Study Manager under Study settings. |
|
| 4 | Click Add study. The study will appear in the list of studies on the study overview page. An e-mail is sent to the Study Manager with an invitation to the newly created study. |
Continue setting up the study
To complete setting up the study, the following steps need to be performed by the Study Manager:
- Invite a Designer that will build the study design in Viedoc Designer.
- Add study site(s).
- Enter the study details under Study settings: sponsor code, Contract Research Organization (CRO) code, reference ID, study type, sponsor type, study phase, therapeutic area, expected number of subjects, and so on.
- Assign a study design to the sites in the study, once the Designer has published a study design.
- Invite users to the different system roles and clinic roles.
- Open the study in Viedoc Clinic and test the study.
These steps are described in more detail in the eLearning lessons under Study Management.
More information
For an overview of the configuration workflow for initiating a study, see Initiating a design.
For a video tutorial that demonstrates how to add a new study in Viedoc Admin, create a simple study design in Viedoc Designer, manage users in Viedoc Admin and enter data as a site user in Viedoc Clinic, see How to set up a study.
