管理用户
本课内容将告诉您Viedoc支持的角色,怎样给用户授予权限并且如何在研究中查看用户的角色以及他们的角色详情。该指南可供研究经理和中心管理员参考。
简介
电子签名的信息
研究经理需要和中心管理员合作,保证所有Viedoc的用户都应知晓:所有在Viedoc中的电子签名都是和传统纸质版手写签字一样具有法律效力。
在Viedoc中,电子签字的目的/意义总是和 Sec. 11.50 of FDA 21 CFR part 11定义的“responsibility”相一致。亦即确认其对已录入数据所承担的责任。 Viedoc会在签字签名完成时保存电子签名的内容,签字时间和签字人的稽查轨迹。
http://help.viedoc.net/l/1648/This is a single-sourced file that should have the following content:
The Study Manager should, in cooperation with the site manager(s), ensure that all users of Viedoc are informed, and certify, that all electronic signatures created in the system are intended to be the legally binding equivalent of a traditional handwritten signature.
In Viedoc the purpose/meaning of a signature is always “responsibility” as used in Sec. 11.50 of FDA 21 CFR part 11. The signer is thereby acknowledging his/her responsibility for the entered data. Viedoc keeps account of what was signed, who signed it and when the signature was performed.
Viedoc中的角色
两种类型的角色
在Viedoc中有两种类型的角色。
- 系统角色是在系统中已经定义的,这些角色可以有权限进入Viedoc Admin或者Viedoc Designer的用户,请见系统角色。
- 临床角色是研究特定的并且可以使用Viedoc Clinic的角色,请见临床角色。
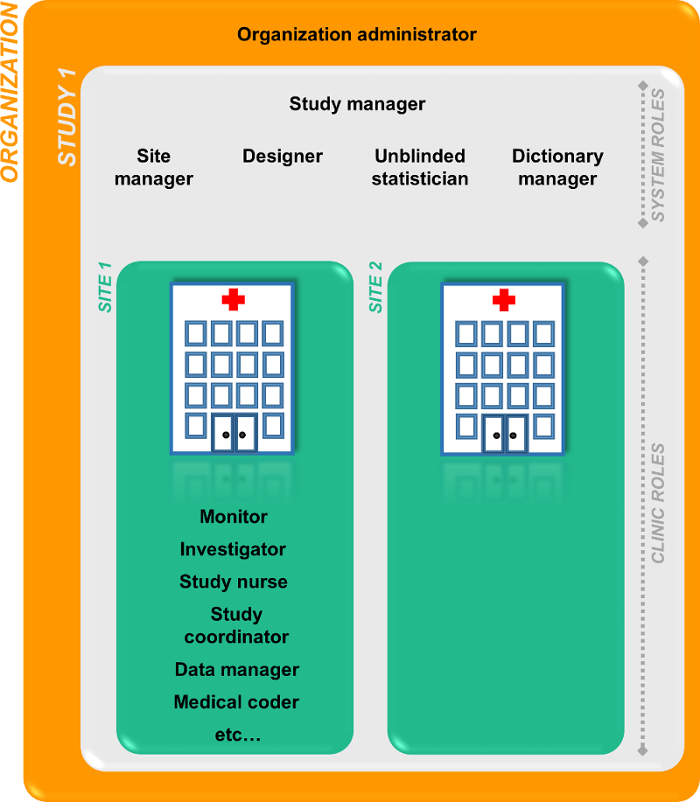
组织管理员可以邀请研究经理。研究经理可以邀请系统角色和临床角色。研究经理也可以把临床角色的管理分配给中心管理员。
系统角色
系统角色是在Viedoc中已经定义好的,无法根据您的研究做修改。系统角色是在Viedoc Admin或Viedoc Designer中可以使用的角色。
请看以下系统角色。
| 角色 | 描述 |
|---|---|
| 组织管理员 | 组织管理员可以管理整个组织中所有的研究项目。组织管理员可以发起一个新的研究并且可以在Viedoc Admin中指派研究经理。 |
| 研究经理 | 研究经理可以为用户添加角色,可以在研究中添加中心也可在Viedoc Admin中将研究设计应用于中心。对于一个传统的临床研究项目,一般研究经理的角色会分配给项目经理。 |
| 设计人员 | 设计人员可以在Viedoc Designer中设计研究。 |
| 中心管理员 | 研究经理在Viedoc Admin中可以指派中心管理员,中心管理员可以指派中心临床用户。通常来说中心管理员可以为CRA添加权限。 |
| 非盲统计师 | 非盲统计师可以在Viedoc Admin中管理随机。这个角色是只有项目需要随机的情况下使用并且可以控制并管理随即表。 |
| 词典管理员 | 词典管理员可以上传医学编码词典。 |
| 参考值源管理员 | 参考值源管理员可以在项目层面上管理参考值源。参考值源管理员也可以把参考值源管理的权力分配给中心管理员。 |
| API管理员 | API管理员可以有权限管理API设置并且可以配置API。完整的API配置指南Viedoc 4 Public API Documentation中,请询问您的Viedoc代表来获得文档的副本。 |
| eTMF管理员 | eTMF管理员在Viedoc Admin中管理eTMF应用。这个角色可以将临床角色映射到eTMF角色,并且拥有管理Viedoc eTMF结构配置的权限。 |
一个组织可以有多个组织管理员。一个研究可以有多个研究经理,设计人员,非盲统计师,词典管理员,参考值源管理员和API管理员。一个中心可以有多个中心管理员。
临床角色
临床角色的权限归属于这些角色,可以在Viedoc Designer中设定这些权限。他们都是研究特定的并且可以有权限进入Viedoc Clinic中。研究经理或中心管理员可以分配这些临床角色给中心用户。每个研究可以有无上限数量的临床角色。
临床角色的例子:
- 研究者
- 研究护士
- 临床管理员
- 数据经理
- 医学编码员
This is a single-sourced file that should have the following content:
1. Two types of roles
2. System roles
3. Clinic roles
The content is shared by the lessons 'Managing Users (OA)" and "Managing Users (STM and SIM)"
研究中的用户
组织/研究/中心中用户的概况
可以在以下三个地方查看到用户列表:
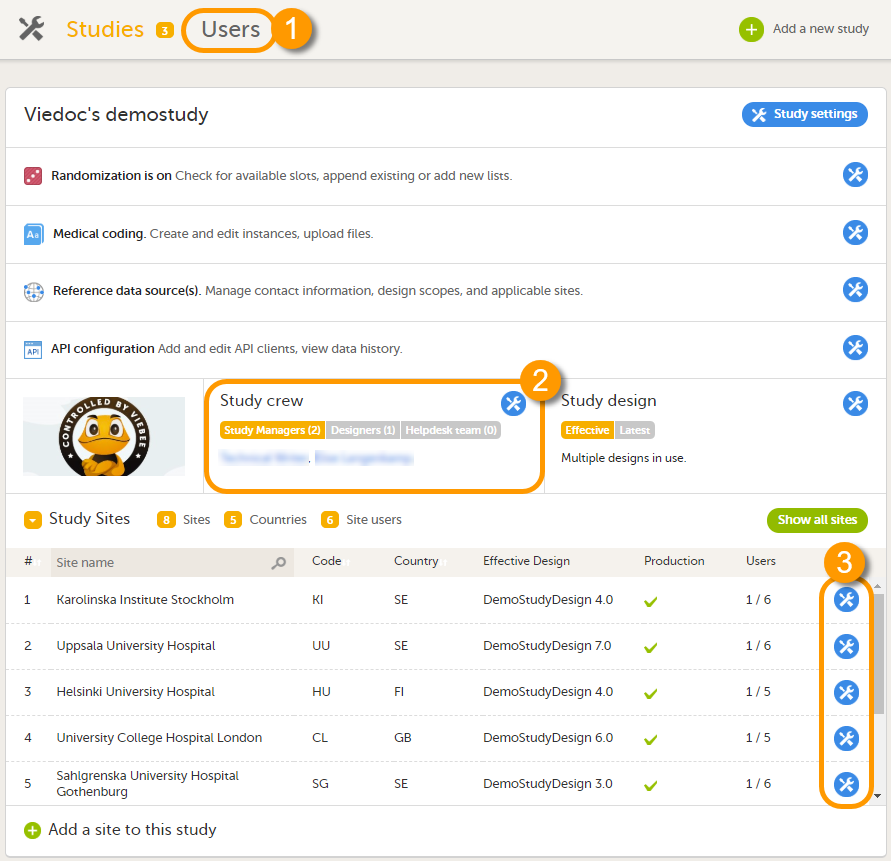
1. 在用户名页面,这张表可以查看到组织中的用户列表。
2. 在临床试验人员窗口中可以查看到研究中的系统角色的用户。
3. 在中心用户栏中可以查看到中心临床角色的用户。
请注意!所有显示的用户列表只有当您有权限管理时才会显示(邀请或移除)。若您是研究经理,您也可以看到组织管理员。若您是中心管理员,您可以看到研究经理。但是这两种情况下您不可移除或邀请这些用户。
用户名
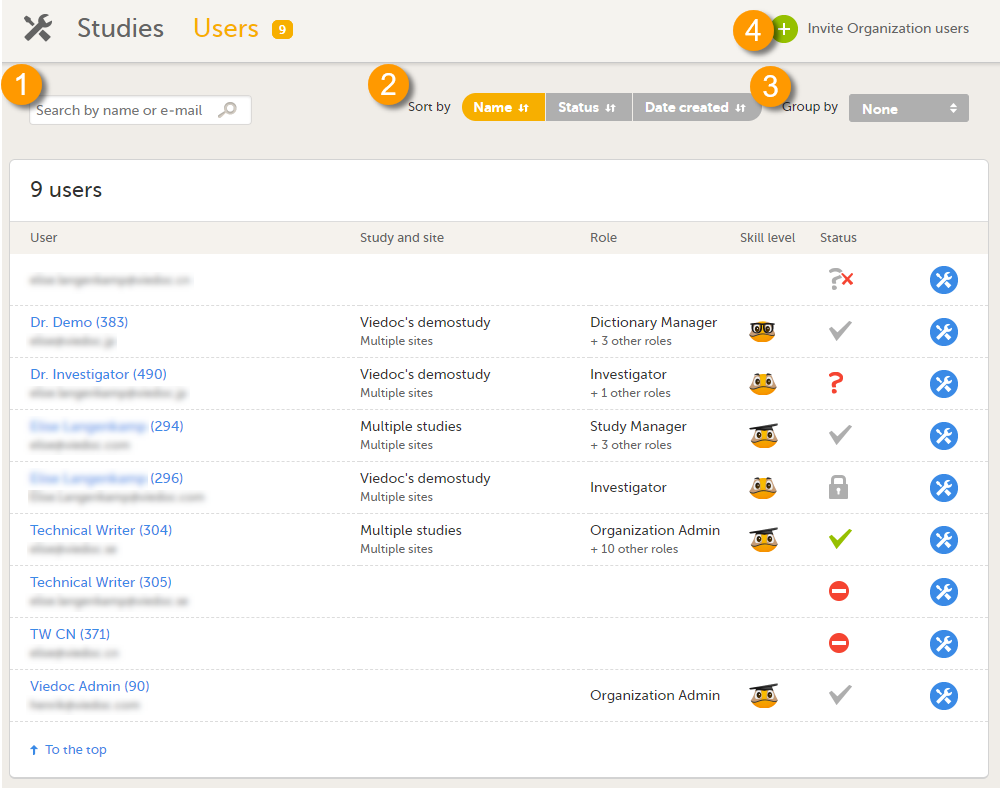
在用户名页面您能查看到组织内所有的用户并且包括以下信息:
- 用户名,
- 用户ID (在用户名后),
- 邮箱地址,
- 用户有权限的中心,
- 用户的角色,
- 用户的Viedoc等级(请见Viedoc的用户等级),
- 用户的状态(请见用户状态)。
如果用户没有被批准的角色,因为邀请仍然在等待,拒绝或者用户已移除,则在页面上只会有用户的邮箱而其他的信息为空白。
在本页,您可(见图):
1. 通过输入用户名和用户邮箱地址来搜索在组织中的特定用户的,
2. 以名字,状态和生成日期来筛选用户,
3. 用户可以以临床试验来归组,
4. 邀请组织用户(只有组织管理员可以),
临床试验研究队伍
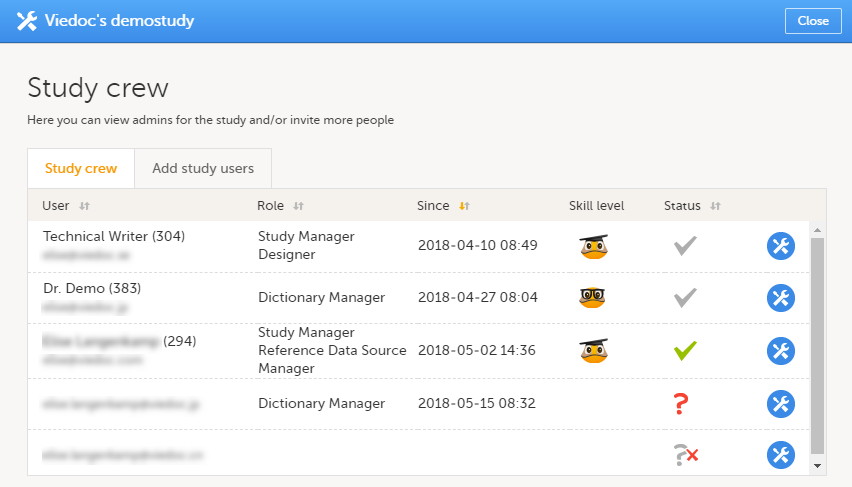
临床试验研究队伍列举了用户的系统角色并且包括以下信息:
- 用户名,
- 用户ID (用户名后),
- 邮箱地址,
- 用户拥有的角色,
- 用户被邀请该角色的日期和时间(如果邀请仍然等待通过)或者用户获得该权限的日期和时间(如果邀请已经被用户通过)*,
- 用户的Viedoc等级(请见 Viedoc用户等级),
- 用户状态 (请见用户状态)。
*如果用户有多个角色,则第一个被邀请或者接收的角色会显示在此处。
您可通过用户名,角色,邀请(接收)日期和状态筛选用户。筛选时只需点击表头而箭头会以升序或降序顺序来排列。
用户状态
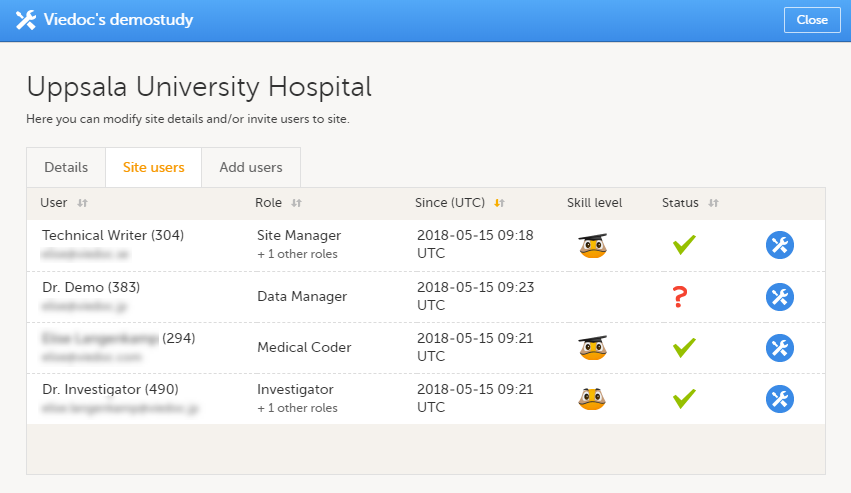
中心设置中的中心用户窗口列举了用户的临床角色并且包括以下信息:
- 用户名,
- 用户ID (用户名后),
- 邮箱地址,
- 用户拥有的角色,
- 用户被邀请该角色的日期和时间(如果邀请仍然等待通过)或者用户获得该权限的日期和时间(如果邀请已经被用户通过)*,
- 用户的Viedoc等级(请见 Viedoc用户等级),
- 用户状态 (请见用户状态)。
*如果用户有多个角色,则第一个被邀请或者接收的角色会显示在此处。
您可通过用户名,角色,邀请(接收)日期和状态筛选用户。筛选时只需点击表头而箭头会以升序或降序顺序来排列。
Viedoc用户等级
Viedoc用户等级会给用户一个使用Viedoc经验的指导。这个等级是和用户登录次数相关的。
| 用户等级 | 图标 | 描述 |
|---|---|---|
| 新手 |  |
≤ 20 登录 |
| 进阶 |  |
21-100 登录 |
| 专业 |  |
101-1000 登录 |
| 高手 |  |
> 1000 登录 |
用户状态
用户的状态在以下列表陈列:
| 状态 | 图标 | 描述 |
|---|---|---|
| 上线 |  |
用户当前登录了Viedoc并且没有等待确认的邀请。 |
| 下线 |  |
用户当前未登录Viedoc并且没有等待确认的邀请。 |
| 等待 |  |
用户至少有一个需要确认邀请的角色。即便用户接受了其他角色的邀请这个问号仍然会存在。 |
| 等待确认的证书 |  |
用户有必须研读的文件但还未确认阅读&理解。 |
| 已拒绝 |  |
用户拒绝了所有角色的邀请并且从未有任何角色在研究中。 |
| 被锁 |  |
用户在Viedoc中被锁定(用户在系统中连续三次输错密码)。 |
| 已移除 |  |
用户之前在研究中有角色但是现在角色已经被移除。 |
在用户名页面(请见用户名),您可能会发现以下情况:
如果用户不以临床试验归组,则用户状态会显示为您所有有权限的项目。因此如果在某个研究中有未确认邀请,则红色的问号和等待的状态会显示。若您根据临床试验归组,则用户的状态会根据该临床试验显示。因此用户可能在这个研究中是等待但是在别的临床试验中是上线状态。
This is a single-sourced file that should have the following content:
1. Overview of users in the organization/study/site
2. The Users tab
3. The Study crew
4. The Site users
5. The user status
The content is shared by the lessons 'Managing Users (OA)" and "Managing Users (STM and SIM)"
用户设置
当您需要查看特定用户的信息,点击工具图标后的名字,您可查看到用户设置窗口弹出。
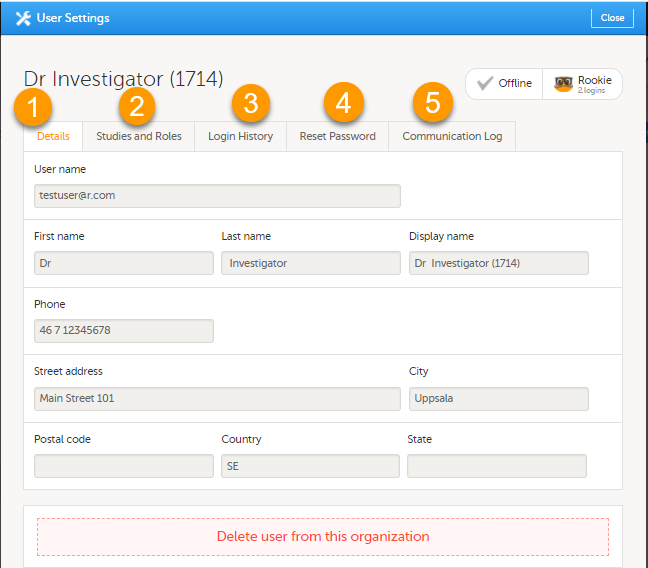
用户设置窗口会显示用户名,用户邮箱,用户ID(括号中),用户状态和用户等级,您可做以下操作:
1. 在详情栏,您可查看用户名和联系信息。
2. 在研究和角色栏,您可查看用户角色和有权限的中心,包括邀请/接受相应角色的时间。角色是由临床试验归组的。您可在角色旁点击垃圾桶图标已移除该角色。
3. 在登录历史栏,您可查看用户的登录记录,包括日期和时间,IP地址和使用的浏览器。显示最多100条登录记录。
4. 在重设密码栏,您可重置用户的密码,如果用户忘记他/她和他/她的安全问题答案。Viedoc将可发送重置密码的链接。
5. 在交流日志标签内,您可以浏览该用户最近20条交流日志的记录并且可以下载该用户的完整版交流日志。下载得到的Excel文档中包含该用户相关的邮件和短信交流信息。所有有权限(研究/中心管理员)访问Viedoc Admin的用户设定标签的用户都有权限下载交流日志。
注意!在Viedoc 4.70版本以前的邮件和短信交流记录也存在,但是没有同等程度的详细信息。
http://help.viedoc.net/l/e5eb09/This is a single-sourced file that should have the following content:
To view the details of a specific user, click the toolbox icon behind the name of that user in any of the previously described user lists. The User Settings window will open.
The User Settings window displays the name and email address of the user, the user ID (in parentheses), the status and the skill level. You can perform the following actions:
1. On the Details tab, you can view the user's name and contact details.
2. On the Studies and Roles tab, you can view a list of all roles and sites the user has access to, including the date and time of invitation/acceptance of that role. The roles are grouped per study. You can delete roles by clicking trash can icon behind that role.
3. On the Login History tab, you can view a list of logins by the user, including date and time, the IP address, and the browser that was used. The number of displayed entries is limited to the latest 100 logins.
4. On the Reset Password tab, you can reset the password for that user, if the user has forgotten his/her password and the answer to his/her challenge question. Viedoc will send a notification to the user with a link to create a new password.
The content is shared by the lessons 'Managing Users (OA)" and "Managing Users (STM and SIM)"
用户报告
您可下载用户和角色的PDF或Excel格式的报告,报告中包括您有权限的中心的所有用户和角色的信息。请见下载用户报告的指南。
注意!
- 只有正式中心及其角色/用户的信息会包括在报告中。
- 日志中将包含研究项目的系统角色(不包括组织层级的用户)。
报告的内容取决于您的系统角色:
| 如果您是一位 | ... 则日志将包括: |
|---|---|
| 组织管理员 |
系统角色中的API管理员,词典管理员,非盲统计师,参考值源管理员和eTMF管理员。 |
| 研究经理 | 系统角色中的API管理员,词典管理员,非盲统计师,参考值源管理员和eTMF管理员以及所有中心和临床试验中的中心用户(比如所有用户和角色的完整报告)。 |
|
中心管理员 |
系统角色中的API管理员,词典管理员,非盲统计师,参考值源管理员和eTMF管理员以及您有权限的中心和其中的用户。 |
用户和角色日志的PDF报告
您可下载用户和角色的PDF格式报告,报告中包括您有权限的中心的所有用户和角色的信息,按照以下章节:
1. 总结 - 激活/失活的角色,激活/失活的用户和参与数据录入的人员都以中心来分组。
- 活跃角色:该中心正在被用户们使用中的角色。
- 失活角色:曾经分配给相关用户,但已经没有任何用户正在使用状态的角色。
- 活跃用户:至少拥有一个活跃角色的用户。
- 失活用户:曾经至少拥有一个该中心的角色,但已经全部被移除,在该中心中没有任何有效角色的用户。
2. 角色 - 每个角色的权限和相应的历史记录都以中心来分组。
3. 各中心的用户日志 - 所有有权限接触数据的用户包括用户活动都以中心来分组。
4. 账户日志 - 上述用户账户的改变记录以中心组来分组。(以用户ID区分)。
用户管理日志的Excel报告
您可下载用户管理日志的Excel报告,报告中包括您有权限的中心的所有用户和角色的信息,用户管理日志包括以下内容:
- 报表信息 - 关于何时由和人生成本日志,一些本研究项目状态相关的概要信息。以及下列更多详细信息:
- 组织名称
- 研究名称
- 正式研究的GUID
- 演示研究的GUID
- PMS 研究:申办方侧的生产研究的GUID
- PMS 研究:申办方侧的演示研究的GUID
- 用户权限日志 - 本列表分行显示每个中心的所有角色的具体用户权限信息,既包括Clinic角色也包括系统角色。关于本列表上其他列具体内容的一些解释如下:
- 中心组 - 代表该用户是通过中心组的方式获得权限邀请。可包括下列值:培训中心,国家或者所有中心。
- 2FA - 代表该用户拥有何种等级的双重验证。可包括下列值:研究等级,账户等级,或者未激活双重验证。
- 最近一次系统登录日期/时间 - 每个用户的最近一次登录系统的信息(仅适用于终端用户,不适用于API客户端用户)
- 认证 - 代表用户是否收到该角色的认证。可包括下列值:是,否或为空(当该角色没有必修培训时)。
- 如果用户签名确认获得了其角色要求的培训的认证,这一列将显示:Certified:Yes。
- 如果用户仅确认阅读并理解,但还未签名确认获得认证,这一列将显示:Certified:No。
- 用户类型 - 代表用户的类型。可能的值有:End User 或 API Client,以说明该用户是可登录系统的终端中户还是一个Web API 客户端角色。
- 用户邀请日志 - 包含待处理邀请和已拒绝邀请信息的列表,包括临床角色和特殊角色。本工作表中的某些列将在此处进一步解释:
- 角色 - 受邀用户的角色。
- 电子邮件地址 - 每个受邀用户的电子邮件地址。
- 现有用户 - 指示受邀用户是否已经在研究项目中拥有其他角色或是新用户。可能值“是”、“否”。
- 初始邀请发送日期/时间 - 涉及每个用户的首次邀请信息
- 发送初始邀请的用户的ID - 用户的数字用户ID
- 用于发送初始邀请的显示名称 - 发送初始邀请时该用户在Viedoc系统中使用的对应用户的显示姓名。
- 用于发送初始邀请的电子邮件地址 - 为受邀用户发送初始邀请的电子邮件地址。
- 邀请重新发送次数 - 邀请被重新发送的次数。
- 最新邀请发送日期/时间 - 涉及每个用户的最新邀请信息。
- 状态 - 邀请状态,可能包括“待处理”、“已拒绝”。
- 拒绝邀请的日期/时间 – 关于每个用户的已拒绝邀请的信息。
- 证书日志 - 用户的证书列表。在 Viedoc 4.65版本之前授予的证书不包含该证书被授予的角色的信息,即,“授权角色”格将为空。
- 总结 - 总结包含各中心用户的国家,中心编号,中心名,激活/失活用户数以及最近一次登录的日期/时间等信息。
- 账户设定日志 - 所有账户设定的变更记录,包括:用户ID,变更记录,用户名和变更日期/时间。
交流日志的Excel报告
有两种不同的交流日志。一个包含指定用户的信息,另一个包含特定研究的信息。
注意!
- 本交流日志不包括任何与受试者相关的通信(Viedoc Me)。
- Viedoc 4.70发布之前的电子邮件和短信交流日志也存在,但这些日志没有同等级别的详细信息。
指定用户的信息
针对指定用户的交流日志包含与研究中指定用户交流的邮件和短消息。
所有可以访问Viedoc Admin中的‘用户设置’的用户(研究/中心经理)都可以查看指定用户的交流日志。交流日志标签包含以下列:
- 日期&时间
- 消息类型
- 状态 - 注意!状态标签表示为Success或Failed,其中Success表示信息已成功由Viedoc发送,Failed表示信息未能由Viedoc成功发送。此外,如果状态为Success,但收件人没有接收到任何信息,则表示出现的问题在于Viedoc外部。若出现此种情况,或者您的状态表示为Failed,请联系您的PS代表。
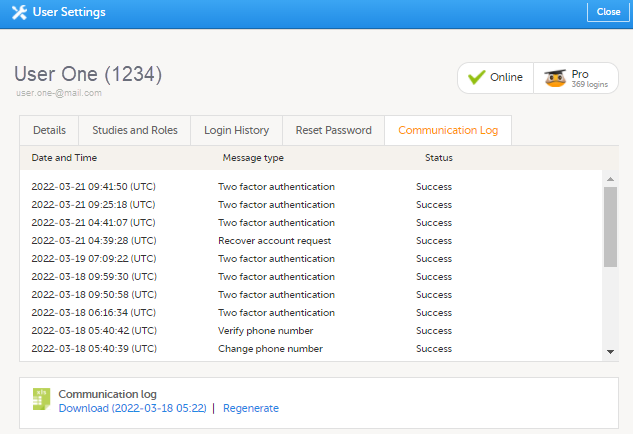
该Excel文件包含一个名为用户交流日志的报表,并在同一Excel报表中包含所有与该研究用户的电子邮件和短信(SMS)通信。
注意! 用户必须激活Viedoc账户并接受至少一个邀请,才能在用户设置窗口的交流日志标签中找到他们的交流记录。
Excel文件中的用户交流日志表包含了指定用户的通信信息 - 这是用户在Viedoc中的活动,与特定研究无关。
- 重置密码
- 验证与通知(更改电话号码/电子邮件地址)
- 2FA (电子邮件/短信)
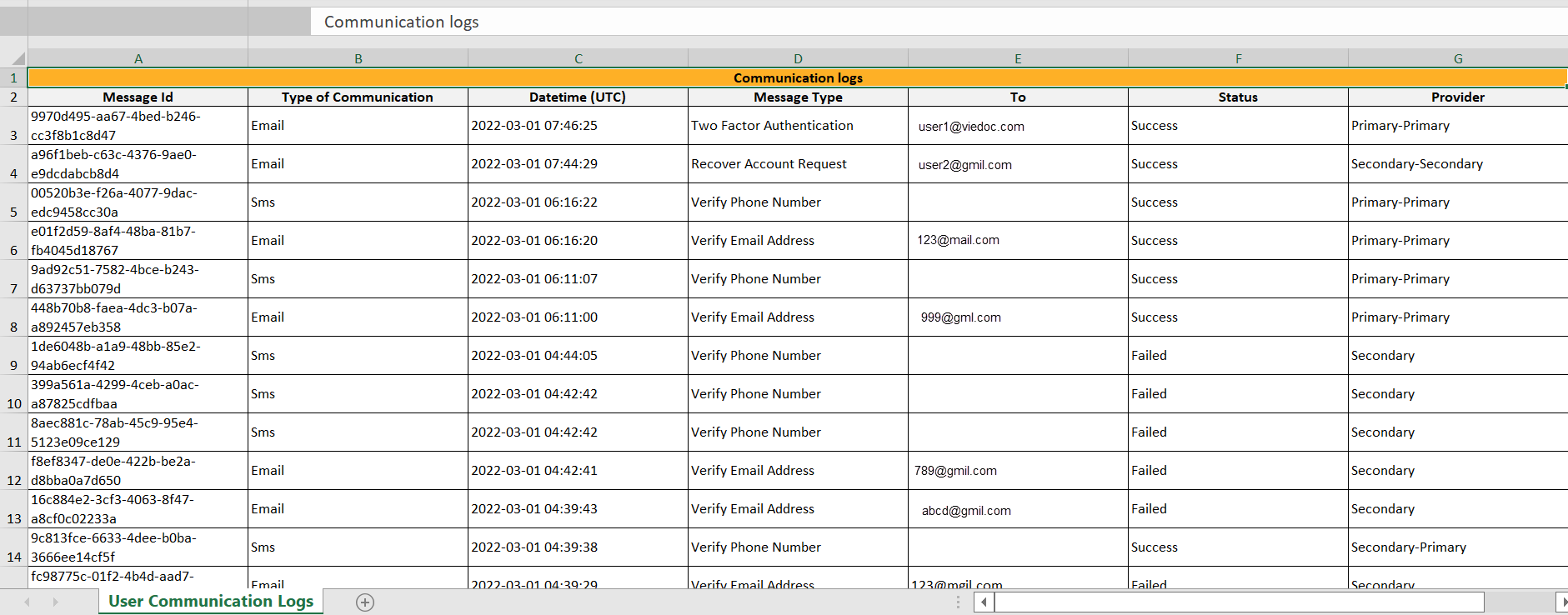
文件名的格式是:UserCommunicationLog-UserID-YYYMMDDhhmmss. (使用UTC)
所有的日志都包含在同一个Excel报表中。该Excel表有以下几列:
| 列 | 描述 |
|---|---|
| 消息ID | GUID: 消息的唯一标识符 |
| 交流类型 | 短信/电子邮件 |
| 日期时间(UTC) | 通信日期和时间 |
| 信息类型 |
通信所涉及的操作:
|
| 收件人 | 信息所发送的电子邮件地址。对于SMS信息,这一列为空。 |
| 状态 | 成功/失败 |
| 供应商 | 供应商名称 - 用于向收件人发送信息的供应商 |
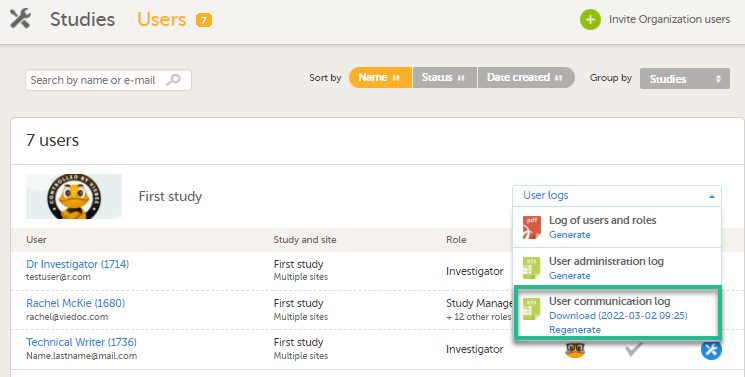
特定研究的信息
在Viedoc Admin中,在用户标签下 - 按研究分组之后,在用户日志的下拉列表中,有一个名为用户交流日志的单独文件,包含下列信息:
该日志仅包含特定研究相关的仅限电子邮件的通信,其中包括:
- 提醒
- 限于研究内的角色的邀请
- 通知(研究访问权限的删除等)
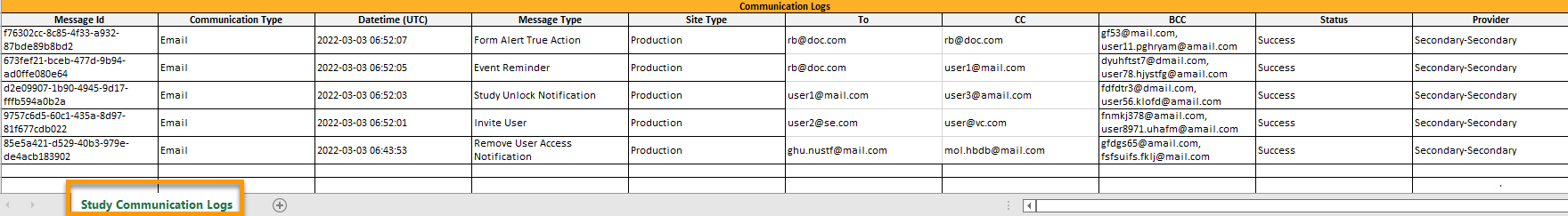
Excel文件包含一个名为研究交流日志的报表。
文件名的格式是:UserCommunicationLog-YYYMMDDhhmmss. (使用UTC)
该Excel表有以下几列:
| 列 | 描述 |
|---|---|
| 消息ID | GUID: 消息的唯一标识符。 |
| 通信类型 | 电子邮件 |
| 日期时间(UTC) | 通信日期和时间 |
| 信息类型 |
通信所涉及的操作:
|
| 中心类型 | 培训/正式(对于邀请和拒绝邀请的信息类型,此栏为空) |
| 收件人 | 电子邮件地址(对于短信,此栏为空。) |
| CC | 抄送的收件人的电子邮件地址 |
| BCC | 密件抄送收件人的电子邮件地址 |
| 状态 | 成功/失败 |
| 供应商 | 供应商名称 - 用于向收件人发送电子邮件的供应商 |
注意!
- 此日志不包括与指定用户相关的信息,比如重置密码、2FA等。
- 此日志文件仅可在Viedoc Admin 中获取。
This is a single-sourced file that should have the following content:
Description of the Users and Roles report in PDF format, that can be downloaded per study from the Users page in Admin.
系统中的中心组
研究经理可以邀请用户进入特定的中心或者是中心组。这些组被称为系统中心组是由系统自动生成的。以下是系统生成的中心组:
- 研究中的所有中心。
- 所有正式版本中的中心包括那些既有正式版又有培训版的中心。
- 国家特定的,比如“瑞典”,包括了该特定国家的所有的正式版中心(包括既有正式版又有培训版的中心)。
当您邀请用户进入系统中的中心组,则用户将自动即时收到该中心组的权限,包括所有在这个中心组中要添加的中心。比如说,您将邀请一个用户进入“匈牙利”,则您将会有所有匈牙利中心的权限。同样的,如果用户在这种中心组的权限被移除,则对于这个中心组所有中心的权限都将被移除。若您需要更多关于系统的中心组的信息,请见Managing Study sites在eLearning中。
http://help.viedoc.net/l/81e4bd/This is a single-sourced file that should have the following content:
The Study Manager can give users access to individual sites, or to a groups of sites at once. These groups of sites are called system site groups and are automatically created by the system, when sites are added to the study. The following systems site groups are created by the system:
All sites, containing all sites in the study.
All production sites, containing all production sites in the study, including the sites that are in both production and training mode.
Country-specific, for example ‘Sweden’, containing all production sites (including the sites that are in both production and training mode) in that specific country in the study.
When you invite users to a system site group, the users will automatically receive instant access to all sites in that group, including all future sites that will be added to that group at a later time. Similarly, these users will automatically lose access to a site, once that site has been removed from the group. For more information about system site groups, see the section Managing study sites in the eLearning.
The content is shared by the lessons 'Managing Users (OA)" and "Managing Users (STM and SIM)"
研究经理的分步指南
在系统中给用户添加系统角色和/或临床角色
只有研究经理才能给用户邀请系统角色。研究经理也可给用户邀请临床角色,或者他/她可以把管理(某些)临床角色的权限委派给中心管理员,请见由中心管理员管理临床角色以获得详细信息。当管理临床角色的权限委派给了中心管理员后,研究经理无法再邀请这些角色的用户了。
当一个用户需要有多中心权限时,最快的方式是通过在临床试验人员的界面邀请(如下描述)。如果一个用户只需要一个中心的权限,您只需在该中心的设置窗口邀请(详见给用户添加临床角色以获得详细信息)。
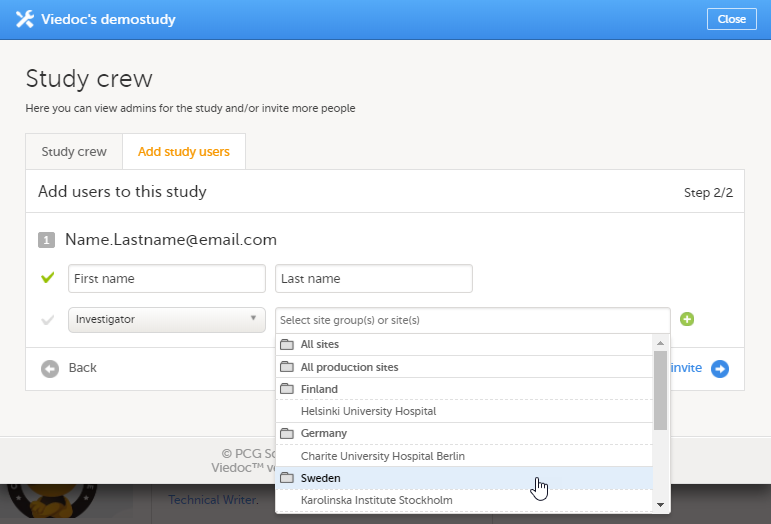
邀请用户时请按照如下步骤。
| 1 | 在Viedoc Admin中,选择您想要邀请用户的研究。 |
| 2 | 点击在临床试验人员格中的工具图标。临床试验研究队伍窗口弹出。 |
| 3 | 在添加研究用户中,输入需要邀请的用户邮箱并点击继续。 提示!您可在此同时添加多个邮箱地址。您只需用分号或者逗号来分隔多个邮件地址。 |
| 4 |
选择被邀请用户的角色。 请注意!I如果任何临床角色已被委派给中心管理员来管理(请见由中心管理员管理临床角色),则该角色不会出现在该下拉菜单。 |
| 5 |
当您选择了中心管理员或者临床角色后,您需要继续选择需要权限的系统中心组或者单个的中心。当您选择了系统中心组后,点击组名(粗体),您可点击中心名来选择单独的中心。
要在演示版中使用与 Viedoc 报标,必须邀请用户有直接的中心权限(不能通过所有中心这个中心组)。对于生产中心,Viedoc 报表可用于所有中心组(所有中心和国家/地区的中心组)。 |
| 6 | 点击发送邀请。 一封邀请邮件将会发送到您添加的邮箱中。 |
重新发送邀请
对于在系统中状态为等待的用户可以重新发送邀请邮件,您可重新在用户名中重发邀请。
重新发送邀请:
| 1 | 在用户名页面,选择您想要重发邀请的用户。点击名字后面的工具图标: 用户设置窗口弹出。 用户设置窗口弹出。 |
| 2 | 在用户设置界面,点击重新发送邀请图标:
新的邀请邮件发出并且:

|
移除用户某个角色的权限
如果您想要移除用户某个角色的权限,您需要询问研究经理,只有研究经理有这个权限。如果研究经理把临床角色的管理委任给了中心管理员,那只有中心管理员可以移除这些角色的权限。
若您想要移除用户的权限,请遵循以下步骤。
| 1 | 在用户名页面,选择您需要移除权限的用户,点击用户名后的工具图标。 用户设置窗口打开。 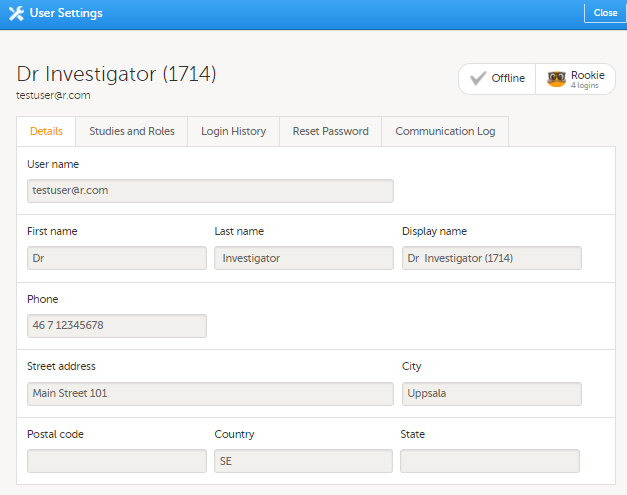 |
| 2 | 在研究和角色栏,滚动到需要被移除权限的研究,中心和角色。点击垃圾箱图标。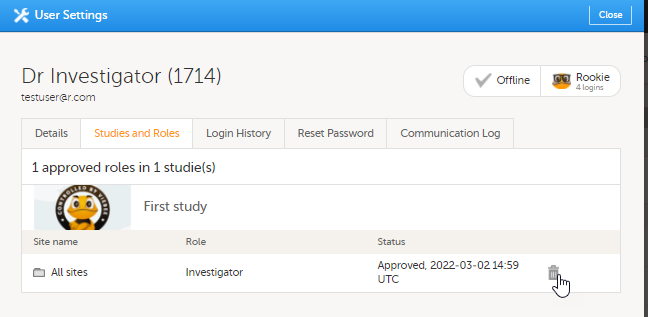 弹出窗口出现。 |
| 3 |
点击删除以确认需要移除的角色,点击撤销可以取消操作。 |
所有用户的记录都会出现在稽查轨迹中即使用户已经被移除。
解锁用户账户
如果一个用户输错三次密码以上,并且忘记了安全问题的答案 - 因此无法使用忘记密码的链接 - 账户将会被锁定。这种情况下研究经理或者中心管理员可以解锁这个账户。
若您需要解锁一个账户,请遵循以下步骤。
| 1 | 在用户名页面, 选择您想要解锁的账户,点击该用户名后的工具图标。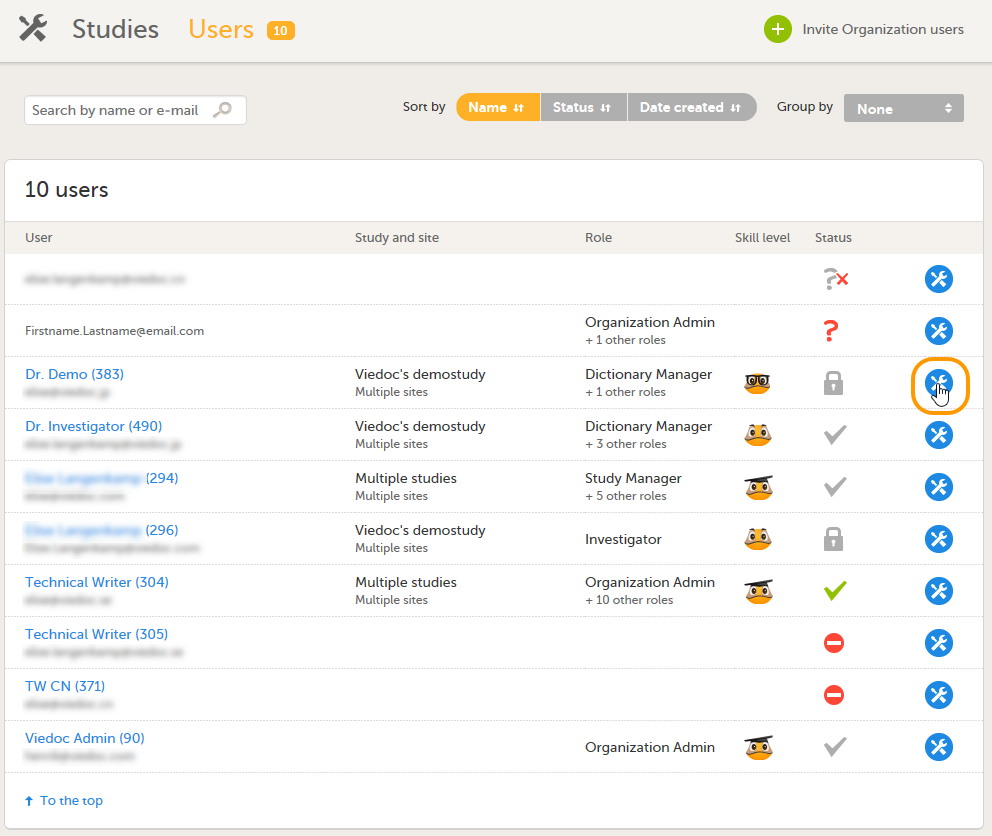 用户设置窗口弹出。 |
| 2 | 在重设密码栏,点击重设密码。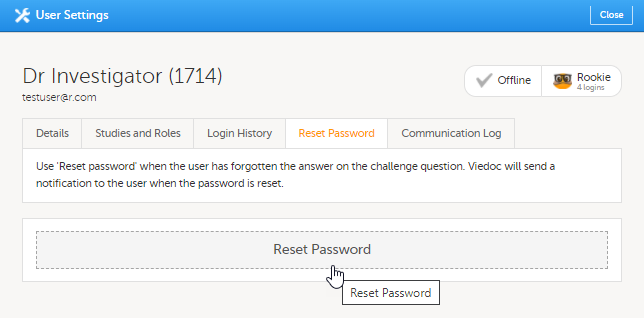 用户将会收到带有重置密码链接的邮件。 |
该链接将会引导用户重置安全问题并且重设密码。安全问题是由用户之前选定的但是问题的答案可以由用户重填而无需和之前一样。
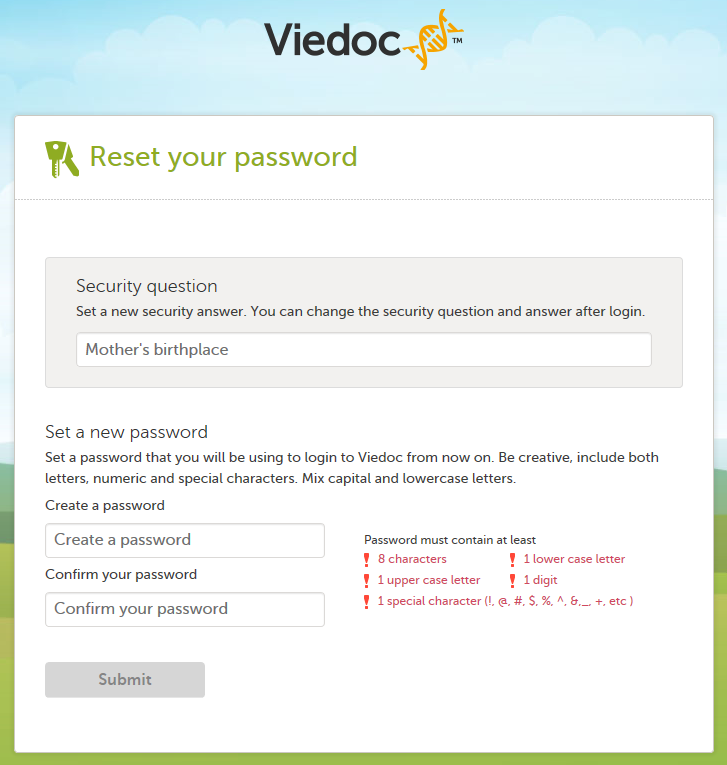
只有重置密码后,用户才可登录并且在安全设置重新选择一个新的安全问题。
请注意!邮箱中的链接用以重置密码只有在12个小时内有效,如果用户在12个小时内没有重置密码,则新的邮件会发送至邮箱中。
由中心管理员管理临床角色
研究经理可以将管理临床角色的权限交予中心管理员。
选中的角色需要由中心管理员管理,请遵循以下步骤。
| 1 | 在Viedoc Admin中,点击研究设置。 研究设置的窗口打开。 |
| 2 | 在设置栏,在由中心管理员管理的临床角色中,您可选择哪些角色需要被中心管理员管理。 这里可以选择的临床角色都是在研究设计中定义的。 |
| 3 | 点击保存后再点击关闭。 |
请注意!这些设置会应用于该研究的所有中心和中心管理员。当(某些)临床角色的管理被委派给中心管理员后,这些角色就不可再被研究经理所管理。
下载用户角色报告
若您需要下载用户角色报告,请遵循以下步骤。
| 1 | 在用户名页面,归组通过选择临床试验。 |
| 2 |
若用户报告之前没有被生成过,点击生成PDF文档或生成Excel文档。若用户报告之前曾被生成过,最近一次生成的版本会被保存在服务器上,同时显示该版本的生成时间和日期。您可以直接点击链接下载已存在的版本,或点击生成生成更新版本。 请注意!用户和角色记录是会根据您使用的语言与之前生成PDF用户使用的语言有关。因此只有当您使用和之前用户相同的语言时,您才可下载旧版本的文档。 |
中心管理员的分布指南
给用户添加临床角色
当研究经理将管理某些临床角色的权限给与中心管理员时,则中心管理员可以邀请用户那些临床角色。
若您需要邀请用户进入某个特定中心时,请遵循以下步骤。
| 1 | 在Viedoc Admin中,点击特定中心工具栏后的图标。 中心设置窗口弹出。 |
| 2 | 在添加用户栏, 输入需要被邀请的用户邮箱并点击继续。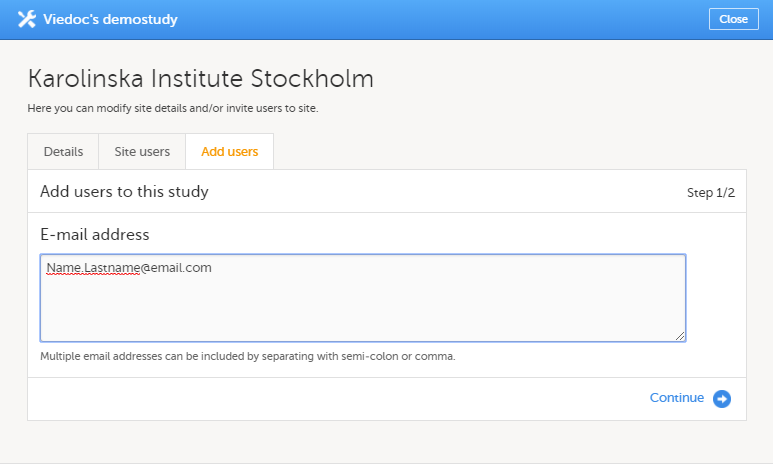 提示!您可以一次邀请多个用户,只需要在邮箱地址中间添加逗号或分号。 |
| 3 | 选择您想要邀请用户的角色。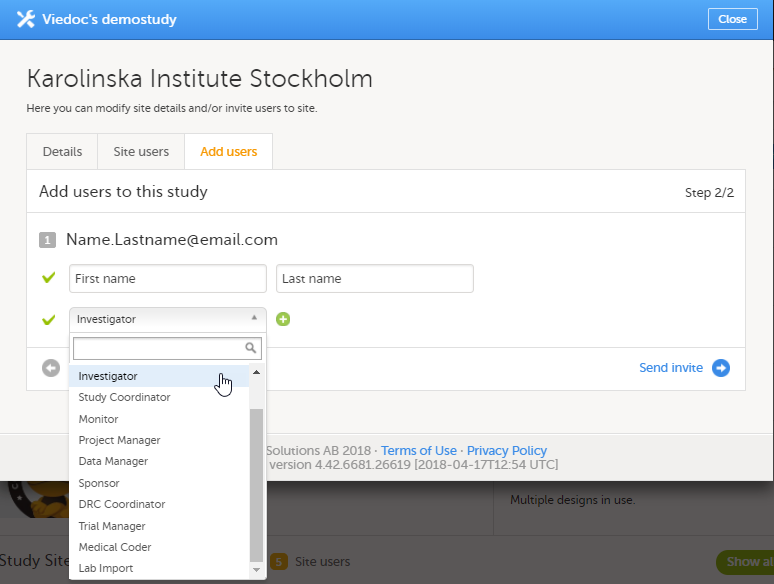 您可添加多个角色只需点击 + 号。新需要添加角色可点击 - 号来移除。 |
| 4 | 点击发送邀请。 一封邀请邮件将会发送到您添加的邮箱中。 |
移除一个用户
点击此处获得移除用户的指南。
解锁一个用户账户
点击此处以获得解锁用户账户的指南。