ユーザーを管理する (組織管理者編)
このレッスンでは、Viedocがサポートしているロールの種類、ユーザーにロールを割り当てる方法、試験にアクセスできるユーザーを確認出来る場所、およびユーザーの詳細について説明します。このレッスンは組織管理者を対象としています。
はじめに
署名に関する重要な情報
サイトマネージャーの協力の下、スタディマネージャーは全Viedocユーザーが、システムで作成する全ての電子署名は従来の手書き署名と法的拘束力が同等であることを意図したものであるとの説明を受け、その旨を保証してもらう必要があります。
Viedocにおける署名の目的・意味は常に、FDA 21 CFR part 11のSection.11.50で言及されている「責任」にあたるものです。従って、署名者は入力されたデータに対する責任を認識していると見なされます。Viedocでは、署名された内容、署名者、署名日時について記録します。
http://help.viedoc.net/l/1648/Viedocのロールについて
2種類のロール
Viedocでは、2種類のロールをサポートしています。
- システムロールはシステムで事前に定義されるロールで、Viedoc AdminまたはViedoc Designer へアクセスすることができます。詳細は、システムロールを参照してください。
- クリニックロールは、試験特有のロールで、Viedoc Clinicへアクセスすることができます。詳細は、クリニックロールを参照してください。
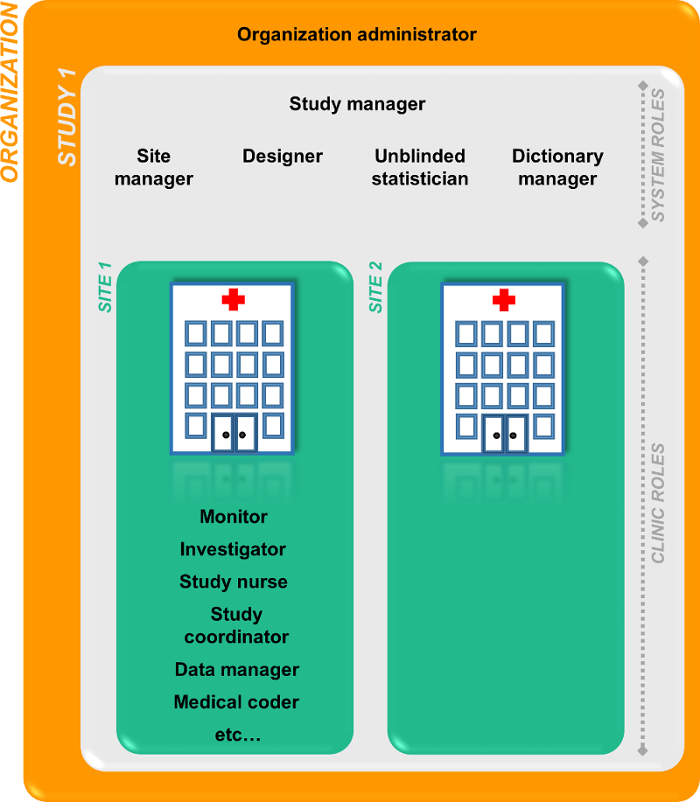
組織管理者がスタディマネージャーを招待します。スタディマネージャーは、ユーザーをシステムロールとクリニックロールに割り当てることができます。スタディマネージャーは、クリニックロールの管理をサイトマネージャーに委任することもできます。
システムロール
システムロールはViedocで事前に定義されており、試験ごとに変更することはできません。システムロールはViedoc AdminまたはViedoc Designerの様々な機能にアクセスすることができます。
次のシステムロールを使用することができます。
| ロール | 説明 |
|---|---|
|
組織管理者 |
組織管理者は、組織内のすべてのプロジェクトを担当します。組織管理者がプロジェクトを開始し、Viedoc Adminですべてのプロジェクトに対してスタディマネージャーを割り当てます。 |
|
スタディマネージャー |
スタディマネージャーはViedoc Adminでユーザーにロールを割り当て、試験に施設を追加し、施設に試験デザインを適用します。一般的に、治験におけるViedoc のスタディマネージャーのロールはプロジェクトマネージャーに割り当てられます。 |
|
デザイナー |
デザイナーはViedoc Designerで試験を構築します。 |
|
サイトマネージャー |
サイトマネージャーはスタディマネージャーによって任命されます。Viedoc Adminを使用して、施設ユーザーにクリニックロールを割り当てます。一般的に、治験におけるViedoc のサイトマネージャーのロールはCRAに割り当てられます。 |
| 非盲検統計学者 | 非盲検統計学者は、Viedoc Adminで割付表を管理します。ランダム化試験において、割付表にアクセスし、管理することができるユーザーを制御する必要がある場合にのみ、このロールが使用されます。 |
| 辞書管理者 |
辞書管理者はコーディング用の辞書をアップロードします。 |
| 基準値データソース管理者 |
基準値データソース管理者は、試験レベルで基準値データソースを管理します。基準値データソース管理者は施設レベルでのデータソースの管理をサイトマネージャーに委任することもできます。 |
| API管理者 | API管理者はAPI設定にアクセスし、APIの設定を行います。API設定の詳細な手順はViedoc 4 Public API Documentationに記載されています。Viedoc 担当者にドキュメントの提供を依頼してください。 |
| eTMF管理者 | eTMF管理者は、Viedoc Admin で eTMF を管理します。eTMFマネージャーはViedoc ClinicのロールをeTMFのロールにマッピングします。また、eTMFマネージャーは、Viedoc eTMFのeTMF構造を管理する権限を持ちます。 |
| デザインインパクトアナリスト | デザインインパクトアナリストはViedoc Adminで改訂の影響分析を実行できます。この権限を持つユーザは、改訂を適用する前に、新しいデザインの改訂が既存のフォームに与える影響を確認できます。 注意!このロールを持つユーザーを招待する前に、デザイン改訂インパクトアナリシスを読んで、デザインインパクトレポートによって、盲検情報を解除してしまう場合について確認することをお勧めします。 |
1つの組織に対して複数の組織管理者を設定することができ、1つの試験に対して複数のスタディマネージャー、デザイナー、非盲検統計学者、辞書管理者、基準値データソース管理者およびAPI管理者を設定することができます。また、1つの施設に複数のサイトマネージャーを割り当てることもできます。
クリニックロール
クリニックロール、およびクリニックロールに属する権限は、Viedoc Designerの試験デザインで設定することができます。クリニックロールは試験特有のロールで、Viedoc Clinicへのアクセスを提供します。クリニックロールはスタディマネージャーまたはサイトマネージャーによって、施設ユーザーに割り当てられます。各試験で使用するクリニックロールの数に制限はありません。
クリニックロールの例:
- 医師
- 治験看護師
- 治験コーディネーター
- データマネージャー
- コーダー
上記の内容は、ユーザーを管理する(組織管理者編)」と「ユーザーを管理する(STM・SIM編)」に共通のものです。
試験ユーザーについて
組織/試験/施設ユーザーの概要
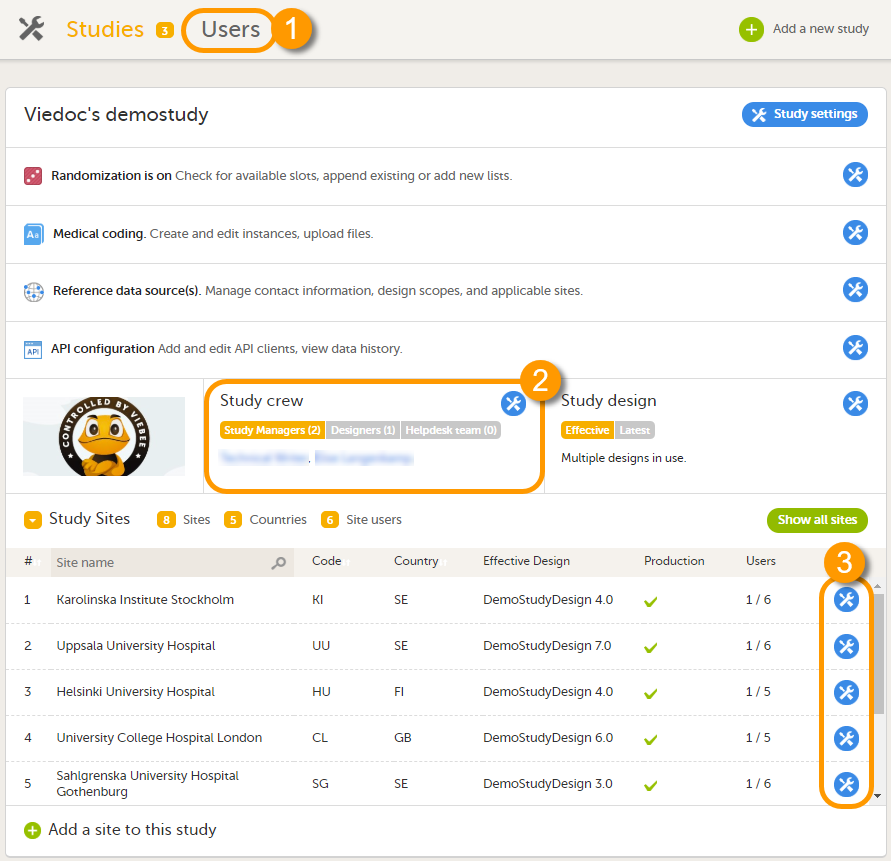
1. ユーザーページ。このページには、組織内の試験のロールに割り当てられているユーザーのリストが表示されます。
2. 試験担当者ウィンドウ。このウィンドウには、試験のシステムロールに割り当てられているすべてのユーザーのリストが表示されます。
3. 施設設定ウィンドウの施設ユーザーのタブ。このタブには、特定の施設内のクリニックロールに割り当てられているすべてのユーザーのリストが表示されます。
注意!3つのユーザーリストにはすべて、ご自身が管理権限(招待または削除)を持っているユーザーとロールのみが表示されます。ご自身がスタディマネージャーの場合は、組織管理者も閲覧できます。サイトマネージャーの場合は、スタディマネージャーも閲覧できます。ただし、どちらの場合も、ユーザーをこれらのロールに招待したり、これらのロールをユーザーから削除したりすることはできません。
ユーザー
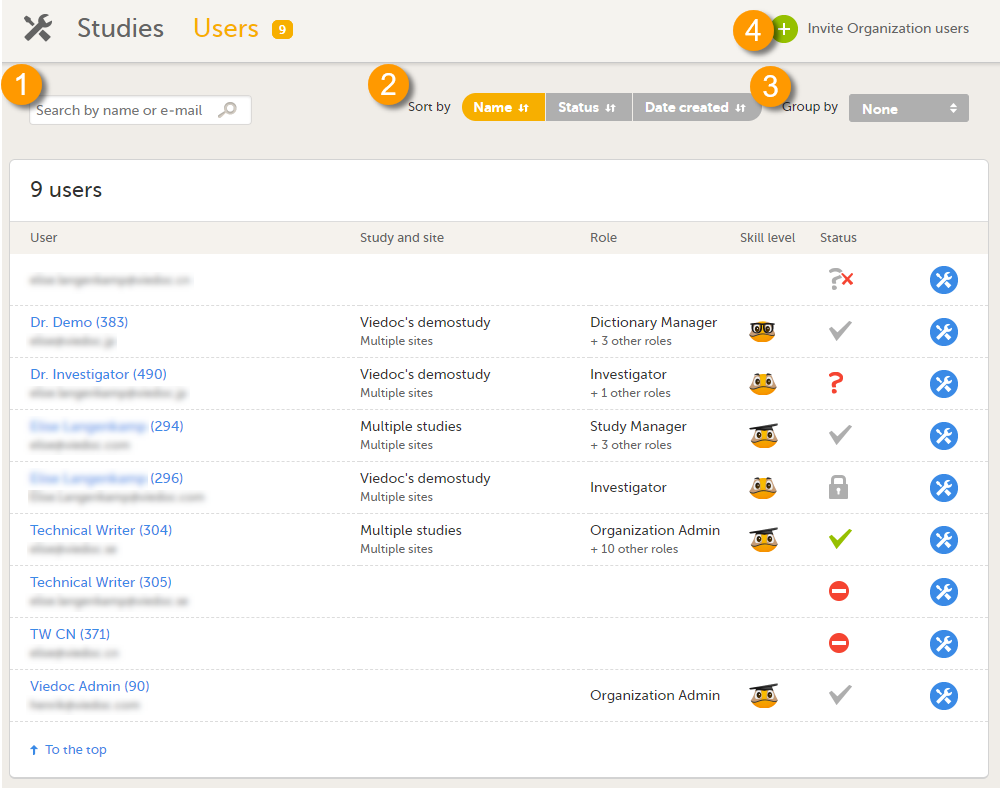
ユーザーページには、組織内のすべてのユーザーの一覧が表示され、次の情報を閲覧することができます。
- ユーザーの名前
- ユーザーID(ユーザーネームの後の括弧内)
- メールアドレス
- ユーザーがアクセスできる試験および施設
- ユーザーに割り当てられたロール
- Viedocユーザーのスキルレベル (Viedocスキルレベルを参照ください)
- ユーザーステータス (ユーザーステータスを参照ください)
招待が保留中または拒否された場合およびロールが削除されたためにユーザーに対して承認済みのロールがない場合は、ユーザーのメールアドレスのみが表示され、他のすべてのフィールドは空のままになります。
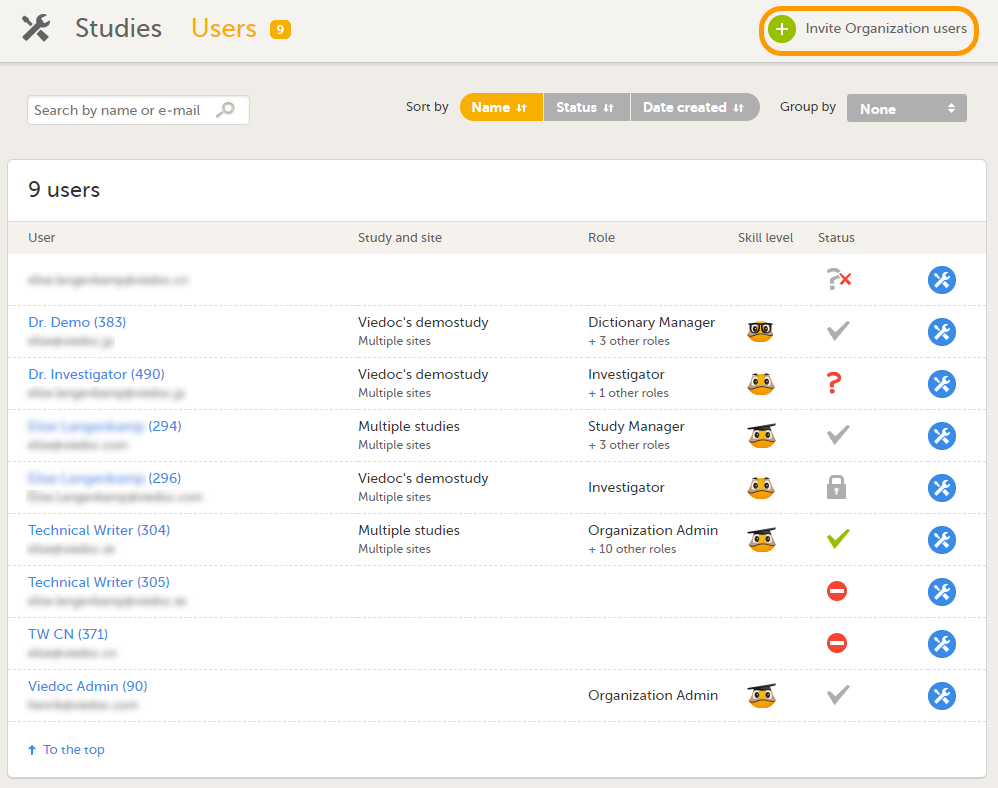
このページでは、以下のことが可能です(画像を参照ください):
1. 検索フィールドにユーザーの名前またはメールアドレスを入力して、組織内のすべてのユーザーの中から特定のユーザーを検索する。
2. ユーザーリストを名前、ステータスまたは作成日で並べ替える。
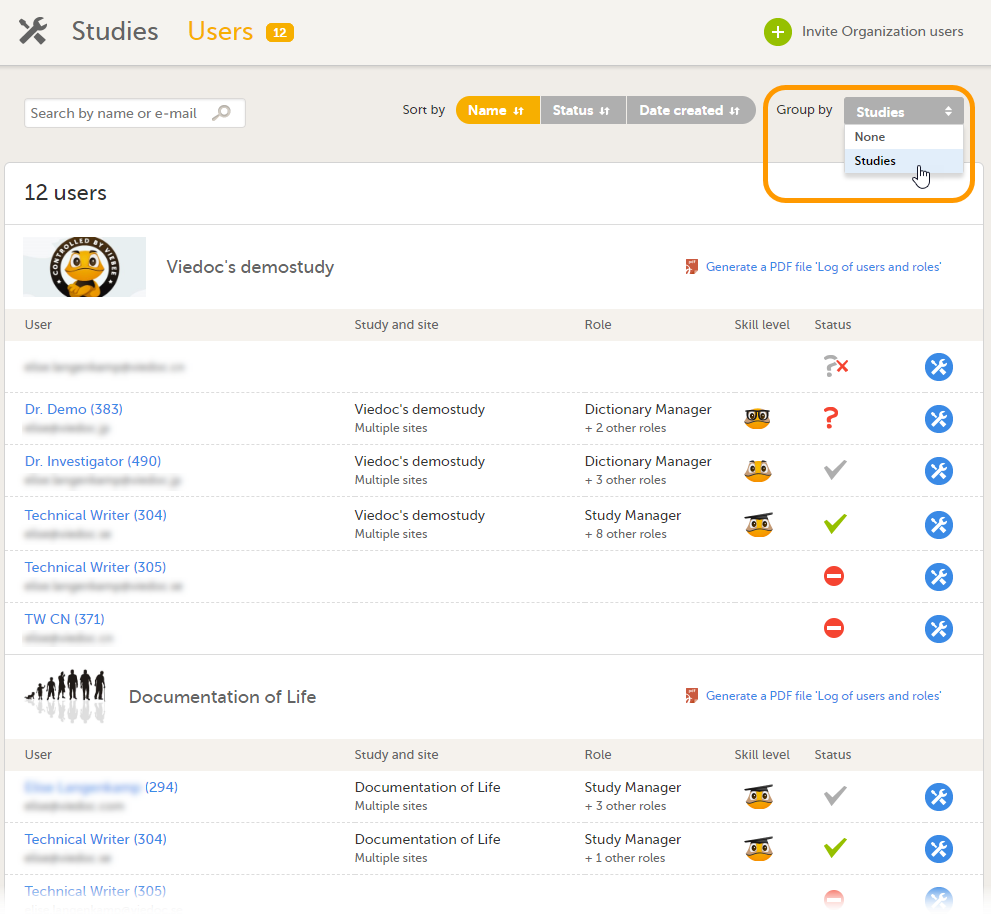
3. グループ化フィールドで試験を選択し、ユーザーのリストをグループ化する。
4. 組織ユーザーを招待する(組織管理者のみ実行可能)。
試験担当者
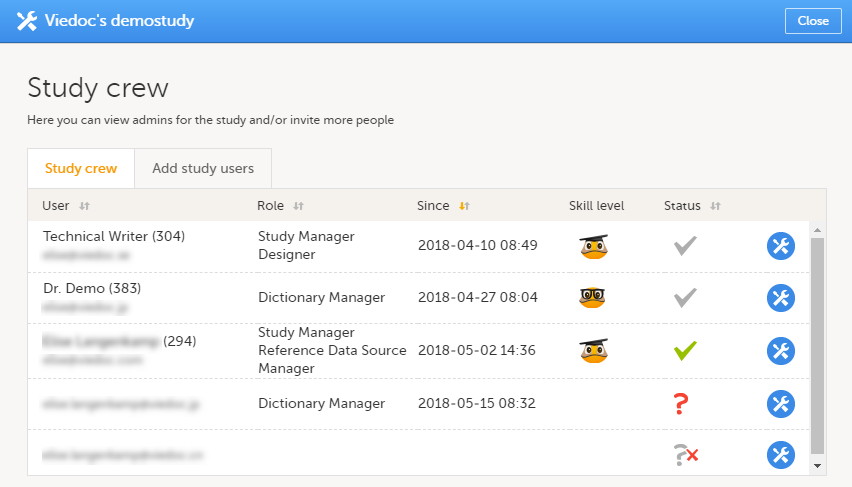
試験担当者ウィンドウには、試験内でシステムロールに割り当てられているすべてのユーザーの一覧が表示され、次の情報が閲覧できます。
- ユーザーの名前
- ユーザーID(ユーザーネームの後の括弧内)
- メールアドレス
- ユーザーに割り当てられたロール
- ユーザーがそのロールに招待された日時(招待がまだ保留中の場合)、またはそのロールにアクセス可能になった日時(招待が承諾された場合)*
- Viedocユーザーのスキルレベル(Viedocスキルレベルを参照ください)
- ユーザーのステータス (ユーザーステータスを参照ください)
*ユーザーが複数のロールに割り当てられている場合、対象施設へのアクセスを許可した最初のロールに対して招待または招待が承諾された日付と時刻が表示されます。
ユーザーは名前、ロール、招待(または招待承諾)の日付およびステータスで並び替えることができます。ユーザーを並べ替えるには、列のヘッダーをクリックするか、列のヘッダーの右側にある矢印をクリックします。ユーザーは昇順または降順で並べ替えることができます。
施設ユーザー
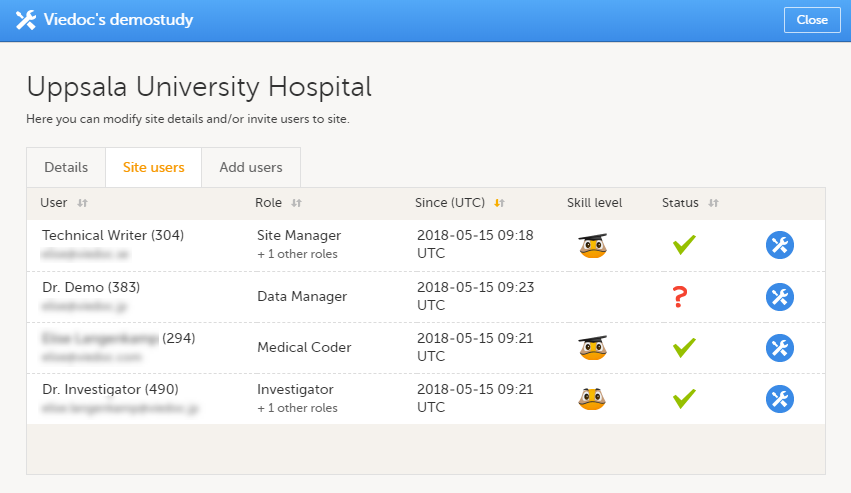
施設設定ウィンドウの施設ユーザータブでは、対象施設へアクセス可能なクリニックロールを持っているすべてのユーザーが表示され、次の情報が閲覧できます。
- ユーザーの名前
- ユーザーID(ユーザーネームの後の括弧内)、
- メールアドレス
- ユーザーに割り当てられたロール
- ユーザーがそのロールに招待された日時(招待がまだ保留中の場合)またはそのロールにアクセス可能になった日時(招待が承諾された場合)*
- Viedocユーザーのスキルレベル(Viedocスキルレベルを参照ください)
- ユーザーステータス(ユーザーステータスを参照ください)
*ユーザーが複数のロールに割り当てられている場合、対象施設へのアクセスを許可した最初のロールに対して招待または招待が承諾された日付と時刻が表示されます。
ユーザーは名前、ロール、招待(または招待承諾)の日付およびステータスで並び替えることができます。ユーザーを並べ替えるには、列のヘッダーをクリックするか、列のヘッダーの右側にある矢印をクリックします。ユーザーは昇順または降順で並べ替えることができます。
Viedocスキルレベル
Viedocスキルレベルは、ユーザーによるログイン回数に基づいたViedoc使用経験値の指標です。
| スキルレベル | アイコン | ログイン回数 |
|---|---|---|
|
ルーキー |
 |
20回以下 |
|
セミプロ |
 |
21~100回 |
|
プロ |
 |
101~1000回 |
|
レジェンド |
 |
1001回以上 |
ユーザーステータス
ユーザーの状態はステータス列に表示されます。
|
ステータス |
アイコン |
状態 |
|
オンライン |
 |
ユーザーは現在Viedocにログインしており、保留中の招待はありません。 |
|
オフライン |
 |
ユーザーは現在Viedoc にログインしておらず、保留中の招待はありません。 |
|
未確定 |
 |
このユーザーには保留中のロールへの招待が少なくとも1件あります。ユーザーがその他のロールへの招待を承諾していた場合でも、クエスチョンマークが表示されます。 |
|
保留中の認証 |
 |
このユーザーには必須ドキュメントが割り当てられていますが、「読んで理解した」が確認されていません。 |
|
リジェクト |
 |
このユーザーはすべてのロールへの招待を拒否しており、試験にアクセスしたことがありません。 |
|
ロックアウト |
 |
このユーザーはViedoc からロックアウトされています(間違ったパスワードを3回続けて入力)。 |
|
削除 |
 |
このユーザーは過去に試験のロールを持っていましたが、現在残っているロールはありません。 |
ユーザーページ(ユーザーを参照)には、以下が適用されます。
試験ごとにユーザーがグループ化されていない場合、ユーザーのステータスシンボルにはご自身がアクセス権限を持っているすべての試験での全体的なステータスが反映されます。つまり、対象のユーザーに対していずれかの試験で保留中の招待が1つある場合、ステータスは未確定となり、赤いクエスチョンマークが表示されます。ユーザーが試験ごとにグループ化されている場合、ステータスシンボルは試験ごとのステータスを反映します。つまり、1つの試験でのユーザーステータスは未確定となり、別の試験ではログイン中になるケースがあります。
上記の内容は、ユーザーを管理する(組織管理者編)」と「ユーザーを管理する(STM・SIM編)」に共通のものです。
Viedoc Adminでのユーザー設定
特定のユーザーに関する詳細を表示するには、先述のユーザーリストのいずれかで、対象ユーザーの名前の右側にあるツールボックスアイコンをクリックします。次に、ユーザー設定ウィンドウが開きます。
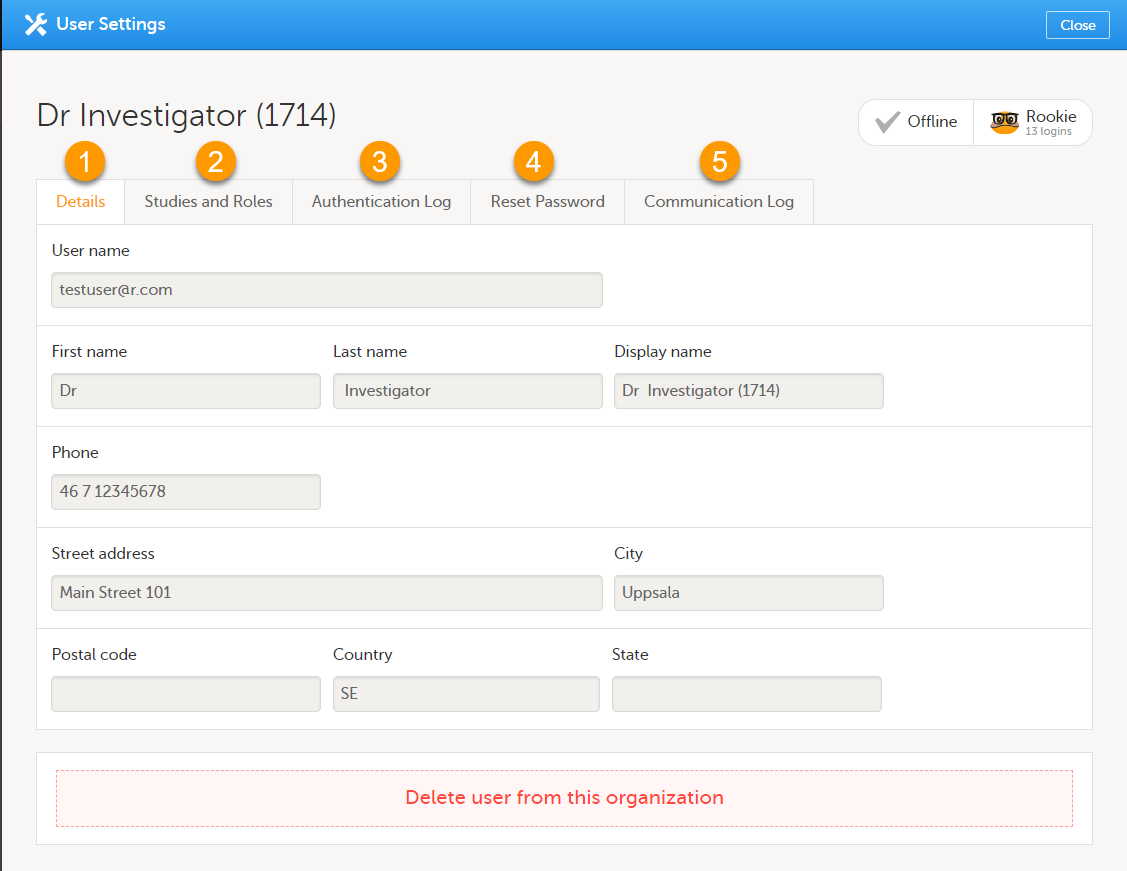
ユーザー設定ウィンドウには、ユーザーの名前とメールアドレス、ユーザーID(括弧内)、ステータスおよびスキルレベルが表示されます。また、次のアクションを実行することができます。
1. 詳細タブでは、ユーザーの名前と連絡先の詳細を閲覧することができます。
2. 試験とロールタブでは、そのユーザーがロールの招待を受け、承諾した日時と、アクセス可能なすべてのロールとサイトのリストを閲覧することができます。ロールは試験ごとにグループ化されています。ロールの横にあるごみ箱アイコンをクリックすると、ロールを削除することができます。
3. 認証ログのタブで、日付と時刻、IPアドレス、および使用されたブラウザを含めた、ユーザーのログインリストを閲覧することができます。表示数は最新のログイン100回までに制限されています。
4. ユーザーがパスワードと秘密の質問への回答の両方を忘れた場合は、パスワードのリセットタブで、そのユーザーのパスワードをリセットすることができます。新しいパスワードを作成するためのリンクが記載された通知がViedocからユーザーに送信されま。
5. コミュニケーションログタブでは、ユーザーの最新20件のコミュニケーションログを表示されます。さらに、試験ユーザーへの電子メールやSMSによるコミュニケーション情報を含む、特定のユーザーの完全なコミュニケーションログをExcelファイルでダウンロードすることができます。Viedoc Adminのユーザー設定へのアクセス権限(スタディ/サイトマネージャー)を持つすべてのユーザーがコミュニケーションログにアクセスできます。
注意! Viedoc 4.70リリース以前の電子メールおよびSMSのコミュニケーションログ情報も得られますが、4.70 リリース以降のバージョンと同等の詳細な情報は提供されません。
http://help.viedoc.net/l/e5eb09/上記の内容は、ユーザーを管理する(組織管理者編)」と「ユーザーを管理する(STM・SIM編)」に共通のものです。
ユーザーレポートについて
各試験の、アクセスできる施設の全ユーザーとロールに関する情報が記載されたユーザーログをPDFおよびExcel形式でダウンロードできます。手順はユーザーログをダウンロードするから参照してください。
注意!ユーザーログに記載されるのは本番運用施設および本番運用施設のロールとユーザーのみとなります。
- Viedoc Adminでは、ユーザーおよび管理ログに記録されるのは、本番用の施設およびその施設に関連するロール/ユーザーのみです。
- 試験におけるシステムロール(※組織ユーザーは対象外)は、ユーザーおよび管理ログに含まれます。たとえば、サイトマネージャーはシステムロールに該当するため、本番用施設のログを生成する際には、デモ施設のサイトマネージャーもログに含まれます。
- グループ別に試験を並べ替え、ユーザとロールのログまたはユーザ管理ログレポートを生成する場合、ページが更新されるまで、新しく生成されたファイルのダウンロードリンクが表示されません。
以下の通り、ご自身のシステムロールによってレポートの内容が異なります。
| ご自身のシステムロール | レポートに含まれる内容 |
|---|---|
|
組織管理者 |
API管理者、辞書管理者、非盲検統計学者、基準値データソース管理者、eTMF管理者 |
|
スタディマネージャー |
API管理者、辞書管理者、非盲検統計学者、基準値データソース管理者、eTMF管理者のシステムロールおよび試験内のすべての施設と施設ユーザー |
|
サイトマネージャー |
API管理者、辞書管理者、非盲検統計学者および基準値データソース管理者、eTMF管理者のシステムロール、およびご自身がアクセスできるすべての施設とそれらの施設のユーザー。 |
ユーザーとロールのログ(PDF形式)
ユーザーとロールのログ(PDF形式)には、ご自身がアクセスできる施設の全ユーザーとロールに関する情報が、以下のようにまとめられています。
- まとめ - 施設毎にまとめられた、有効/無効ロール、アクティブ/アクティビティがないユーザー、およびデータ貢献者の概要を、施設ごとに1つのセクションにまとめています。
- 有効ロールとは、ある施設に対して、すべてのアクティブなユーザーが現在持っている明確なロールのことです。
- 無効ロールとは、以前に割り当てられたが、現在アクティブなユーザーがいないロールです。
- アクティブユーザーとは、少なくとも1つの有効ロールを持つユーザーです。
- アクティビティがないユーザーとは、ある施設で少なくとも1つのロールを持っていたが、その施設のすべてのロールが取り消されたユーザーのことである。
- ロール - 施設毎のロールの権限と履歴
- 施設毎のユーザー履歴 - 施設毎のユーザー履歴とユーザーアクティビティ
- ユーザーアカウント履歴 - ログの上記セクションに記載されているユーザーのすべてのユーザーアカウントの変更履歴を、ユーザーごとにグループ化したリスト(ユーザーIDで識別)
ユーザー管理ログ(エクセル形式)
ユーザー管理ログには以下が含まれています。※英語表記となっています。
- Report Info - ログがいつ、誰によって生成されたかという一般的な情報と、試験ステータスに関するいくつかの情報です。以下の詳細情報が含まれます
- 組織名
- 試験名
- 本番試験 GUID
- デモ試験 GUID
- PMS 試験の場合:スポンサー側の本番試験 GUID
- PMS 試験の場合:スポンサー側のデモ試験 GUID
- User Access Log - 施設とロールごとに1行で表示される、ユーザーアクセスに関する詳細情報の一覧です。クリニックのロールおよびシステムのロールも含まれています。このシートの一部の列については、以下でさらに説明します。
- Site Group - ユーザーが施設グループの招待によってサイトへのアクセスを許可された場合に表示されます。設定可能な値は、Training sites、Countries、All sitesです。
- 2FA - 2段階認証のレベルを表示します。設定可能な値は、Study level、Acount level、No two-factor authentication enabledです。
- Latest system login date/time - 各ユーザーの最新のログインに関する情報です(エンドユーザーのみが対象であり、APIクライアントユーザーは含まれません)。
- Certified - ユーザーがロールに対して認定されているかを示します。可能な値はYes、No、または必須のトレーニングセクションがないロールの場合は空白セルです。
- ユーザーがロールに関連する認定に署名している場合、列には「Certified: Yes」と表示されます。
- ユーザーが「Read & Understood(読み、理解しました)」を選択したが、関連する認定に署名していない場合、列には「Certified: No」と表示されます。
- User type -これにより、ユーザーがシステムにログインできるエンドユーザーか、ロールを使ってAPIにアクセスできるWeb APIクライアントかを示します。
- User Invitation Log - 保留中の招待状と拒否された招待状に関する、クリニックのロールや特別なロールを含む情報の一覧。このシートのいくつかの列については、以下でさらに詳しく説明します。
- Role - 招待されたユーザーのロール。
- Email Address - 招待された各ユーザーのメールアドレス。
- Existing User - 招待されたユーザーがすでに試験で別のロールを持っているか、新規ユーザーであるかを示します。 可能な値は はい、いいえ です。
- Initial Invitation Sent date/time - 各ユーザーの初回招待に関する情報。
- Initial Invitation Sent By ID - ユーザーの数値ユーザーID。
- Initial Invitation Sent By Display Name -Viedocでユーザーを識別するために使用された表示名で送信された最初の招待状。
- Initial Invitation Sent By Email Address - 招待されたユーザーに送信された最初の招待のメールアドレス。
- Invitation Resend Count - 招待が再送された回数。
- Latest Invitation Sent date/time - 各ユーザーの最新の招待に関する情報。
- Status - 招待のステータス。Pending、Rejected。
- Invitation Rejected date/time - 各ユーザーが招待を拒否した日時に関する情報。
- Certification Log - ユーザーごとの認証のリストです。リリース4.65以前に実施された認証には、その認証がどのロールに適用されるかについての情報がありません。つまり、列Certified With Rolesのセルは空です。
- Summary - 国、施設コード、施設名、アクティブ/非アクティブユーザー数、最後にアクセスが変更された日時などの情報を含む、施設ごとのユーザーの概要。
- Account Settings Log - すべてのユーザーアカウントの設定変更のリストを、ユーザーID、変更ログ、ユーザー名、日付/時間で表示します。
コミュニケーションログ (Excel形式)
2種類のコミュニケーションログがあります。1つはユーザーに固有の情報を含むもので、もう一つは、試験に固有のコミュニケーション情報を含むものです。
注意!
- このコミュニケーションログには、被験者に関するコミュニケーション情報(ViedocMe)は含みません。
- Viedoc 4.70リリース以前の電子メールおよびSMSのコミュニケーションログ情報も得られますが、バージョン4.70以降のバージョンと同様の詳細な情報は提供されません。
ユーザー固有情報
ユーザー固有のコミュニケーションログは、試験ユーザへの電子メールおよびSMSによるコミュニケーションの情報を含みます。
Viedoc Adminのユーザー設定へのアクセス権限(スタディ/サイトマネージャー)を持つすべてのユーザーがユーザーに固有のコミュニケーションログにアクセスできます。コミュニケーションログには以下のタブが含まれます。
- 日時
- メッセージタイプ
- ステータス - 注意 ! ステータス・ラベルはSuccessまたはFailedで、SuccessはViedocからのメッセージ送信が成功したことを意味し、FailedはViedocからのメッセージ送信に失敗したことを意味します。また、ステータスがSuccessであるにもかかわらず、受信者がメッセージを受け取らなかった場合は、Viedocの外部に問題があることを意味します。このような場合、またはステータスがFailedになった場合は、PS担当者にご連絡ください。
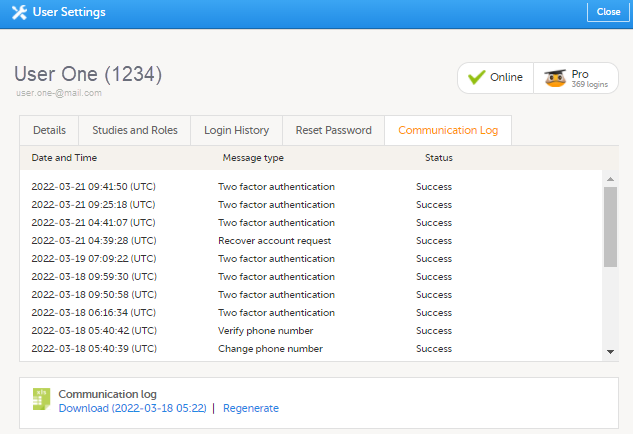
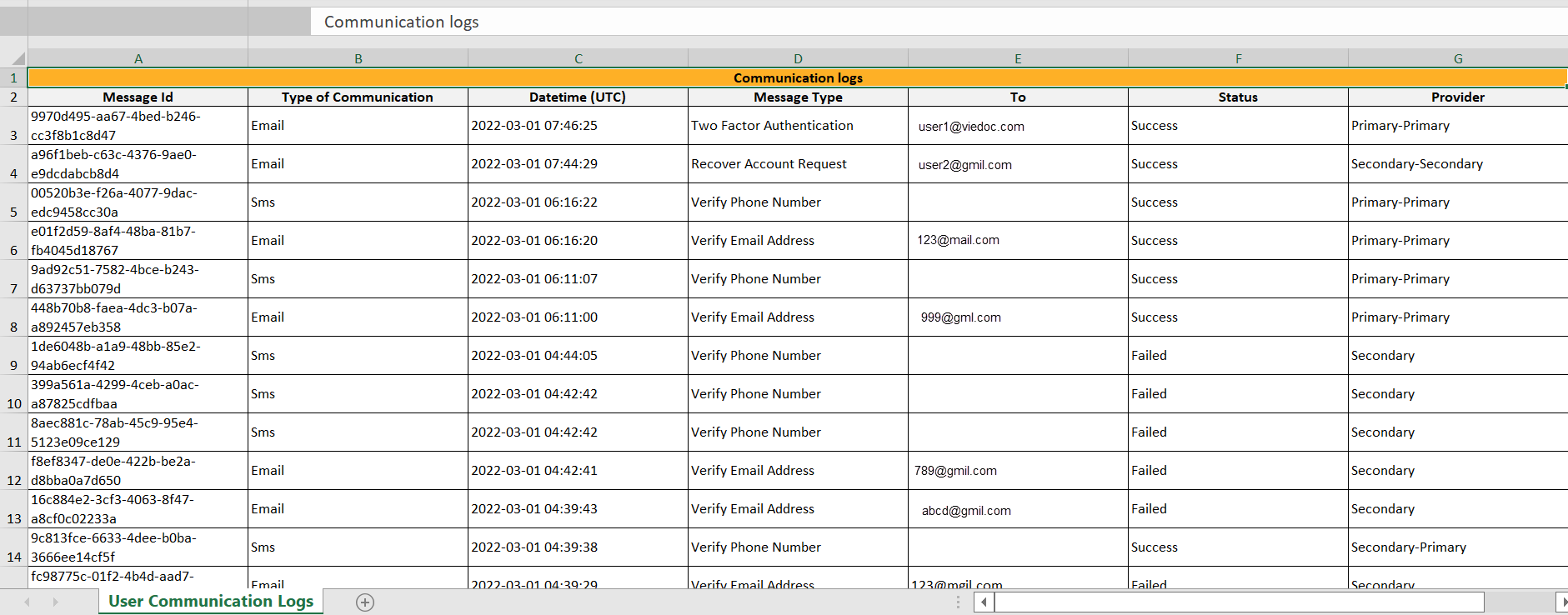
Excelファイルには、User Communication Logsという名前のシートがあり、同じExcelシートにスタディユーザーへのすべての電子メールとテキストメッセージ(SMS)の通信情報が含まれています。
注意! ユーザー設定ウィンドウのコミュニケーションログタブに通信内容が記録されるようにするには、ユーザーはViedocアカウントを有効化し、少なくとも1つの招待をっ承認しなければなりません。
ExcelファイルのUser Communication Logsシートには、ユーザー固有のコミュニケーションに関する情報が含まれています。これは、特定の試験に関係するものでない、Viedocでのユーザーの活動です。
- パスワードのリセット
- 認証と通知(電話番号/メールアドレスの変更)
- 2FA (電子メール/SMS)
ファイル名の形式は UserCommunicationLog-UserID-YYYYMMDDhhmmss. (UTCを使用)です。
すべてのログは、同じExcelシートに含まれています。Excelシートには以下の列があります。
| 列 | 説明 |
|---|---|
| Message ID | GUID:メッセージ固有の識別子 |
| Type of Communication | SMS/電子メール |
| Datetime (UTC) | コミュニケーション日時 |
| Message Type |
コミュニケーションが関係する処理:
|
| To | メッセージが送られた電子メールアドレス。SMSの場合は空です。 |
| Status | Success/Failed。メッセ―ジの送信が成功したか失敗したかを示します。 |
| Provider | プロバイダ名 - メッセージの送信に使用されたプロバイダの名前です。トラブルシューティングに使用されます。 |
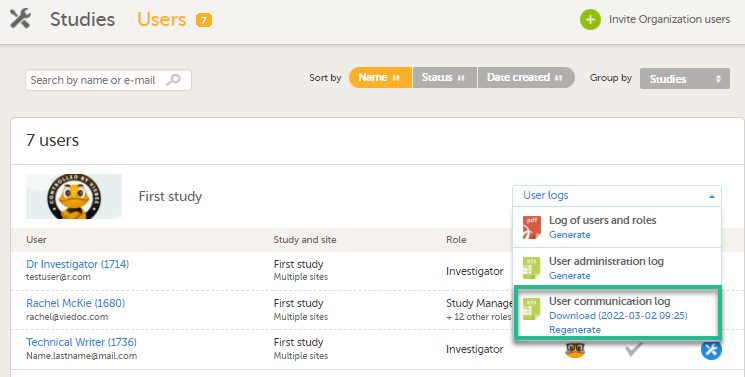
試験固有情報
Viedoc Admin の ユーザー ページで、試験でグループ化します。ユーザーログプルダウンメニューからユーザーコミュニケーションログを選択することで、以下にリストされた情報を含む UserCommunicationLog ファイルをダウンロードできます。
このログには、試験に固有のコミュニケーション情報が記載され、以下のメッセージに関連する電子メールに関する情報のみ含まれます。
- アラート
- 試験内の特定のロールへの招待
- 各種通知 (試験へのアクセス削除等)
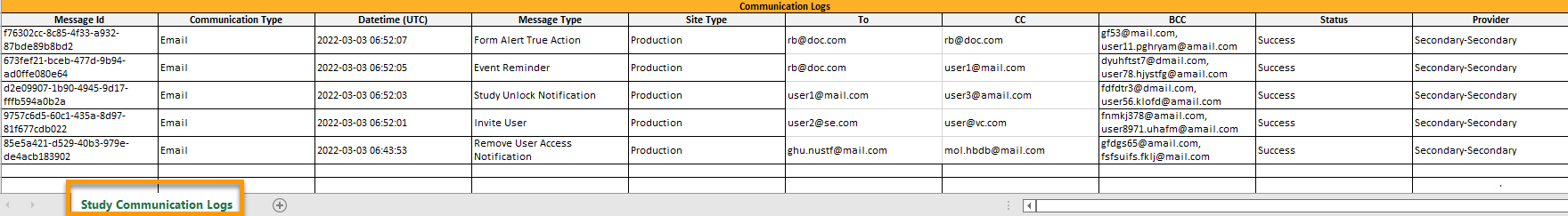
Excelファイルには、Study Communication Logsという名前のシートがあります。
ファイル名のフォーマット: UserCommunicationLog-YYYYMMDDhhmmss(UTCを使用)
エクセルシートには以下の列があります。
| 列 | 説明 |
|---|---|
| Message ID | GUID:メッセージ固有の識別子 |
| Communication Type | Email: 電子メール |
| Date time (UTC) | コミュニケーション日時(UTC) |
| Message Type |
コミュニケーションが関連するアクション:
|
| Site Type | Training/Production (招待、招待の拒否 メッセージでは、空白になります。) |
| To | 送信先電子メールアドレス( SMS メッセージの場合は空白になります。) |
| CC | コピー送信先のメールアドレス |
| BCC | ブラインドコピーの送信先の電子メールアドレス |
| Status | Success/Failed :メッセ―ジの送信が成功したか失敗したかを示します。 |
| Provider | プロバイダ名 - 電子メールの送信に使用されたプロバイダの名前です。トラブルシューティングに使用されます。 |
注意!
- このログは、パスワードのリセットや2ファクタ認証などの、ユーザーに固有の情報は含みません。
- このログはViedoc Admin でのみ入手可能です。
システム施設グループについて
スタディマネージャーはユーザーに個々の施設、または一連の施設へのアクセスを与えることができます。これらの一連の施設はシステム施設グループと呼ばれ、施設が試験に追加されると自動的にシステムによって作成されます。システムによって作成されるシステム施設グループは以下の通りです。
- 全ての施設:試験対象のすべての施設
- 本番環境の全ての施設:本番モードとトレーニングモードの両方が存在する施設を含む、試験対象のすべての本番運用施設
- 国特有: 試験対象の特定の国(例:スウェーデン)における、すべての本番環境の施設(本番モードとトレーニングモードの両方が存在する施設を含む)
システム施設グループにユーザーを招待すると、そのグループに後から追加されるすべての施設を含め、ユーザーは自動的にそのグループ内のすべての施設へ即時アクセスできるようになります。たとえば、ユーザーを「ハンガリー」の国に招待すると、そのユーザーはハンガリー国内のすべての施設へのアクセス権限を取得します。同様に、システム施設グループに招待されたユーザーは、ある施設がそのグループから削除されると、その施設へのアクセスを自動的に失います。システム施設グループの詳細については、eラーニングの試験施設を管理するのセクションを参照してください。
http://help.viedoc.net/l/81e4bd/上記の内容は、「ユーザーを管理する(組織管理者編)」と「ユーザーを管理する(STM・SIM編)」に共通のものです。
組織管理者向けのステップ・バイ・ステップガイド
組織管理者を割り当てる
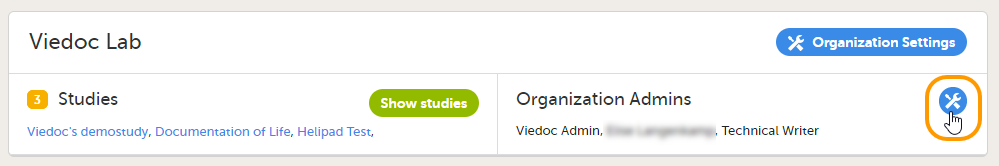
デフォルトでは、各組織に少なくとも1名の組織管理者が存在します。組織管理者をさらに追加する場合、追加操作は組織管理者のみによって実行可能です。
組織管理者を追加するには:
| 1 |
Viedoc Adminの組織ウィンドウで、組織管理者フィールドのツールボックスアイコンをクリックします。
|
| 2 | 管理者を追加のタブで、組織管理者のロールに招待するユーザーのメール電子メールアドレスを入力します。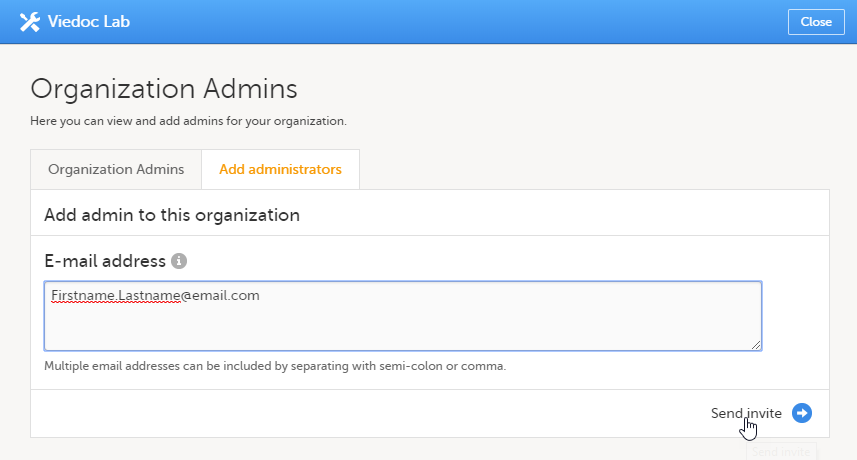 ヒント!このフィールドに複数の電子メールアドレスを追加することにより、一度に複数のユーザーを招待することができます。電子メールアドレスはセミコロンまたはカンマで区切ります。 |
| 3 |
招待を送信をクリックします。 |
ユーザー画面を使用して、ユーザーを組織のロール(組織管理者、eラーニング管理者、および組織レベルのデザイナー)に割り当てることもできます。
| 1 |
ユーザー画面で、組織ユーザーの招待をクリックします。
|
| 2 |
招待するユーザーの電子メールアドレスを入力し、招待するロールを選択します。追加されるロールを選択フィールドをクリックすると、複数のロールを追加することができます。
|
| 3 |
招待を送信をクリックします。 |
組織レベルのデザイナーには、組織内のすべての試験に対するViedoc Designerへのアクセスとプライベートデザインセクションへのアクセス権が与えられます。以下の画像を参照してください。
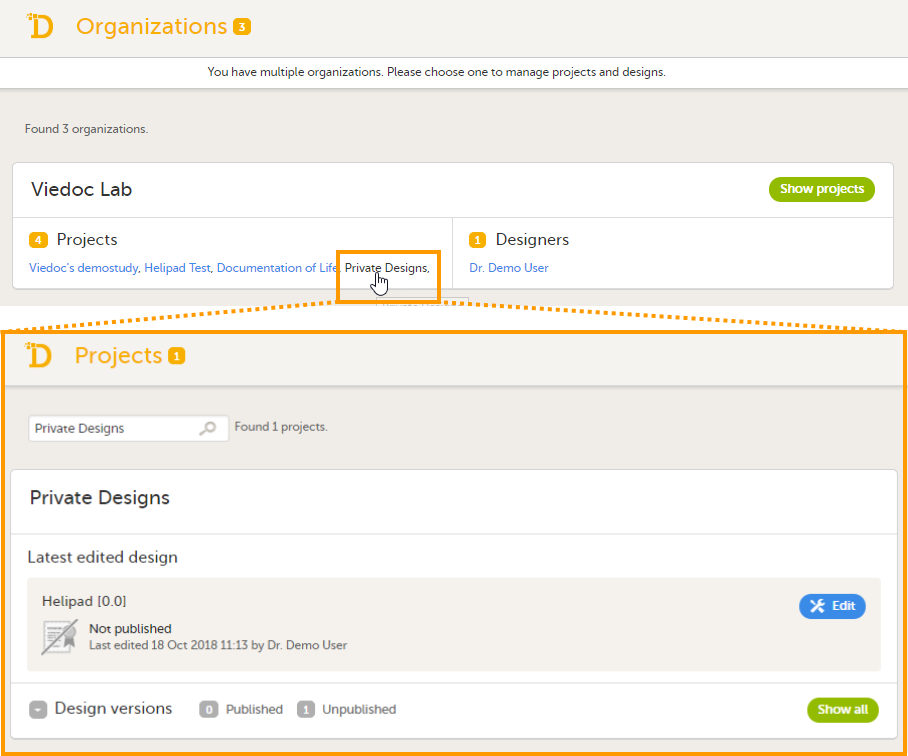
eラーニング管理者を割り当てる
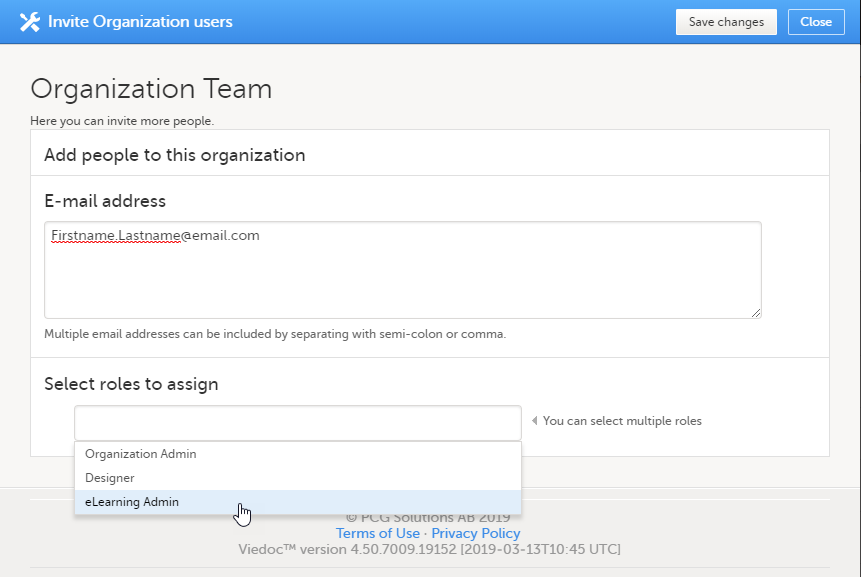
ユーザーページからeラーニング管理者を割り当てるには:
| 1 |
ユーザー画面で組織ユーザーを招待をクリックします。
|
| 2 |
招待するユーザーの電子メールアドレスを入力し、そのユーザーを招待するロールを選択します。追加されるロールを選択のフィールドをクリックすると、複数のロールを追加することができます。
|
| 3 | 保存をクリックします。 指定した電子メールアドレスに招待メールが送信されます。 |
eラーニング管理者のロールに割り当てられると、そのユーザーはViedoc eラーニングのプラットフォームにアクセスすることが可能になり、所属している組織を対象にカスタマイズしたユーザードキュメントを作成することができます。eラーニング管理者の権限を持つユーザーの場合、Viedoc Clinicの試験選択画面に以下のアイコンが表示されます。このアイコンからViedoc eラーニングのプラットフォームにアクセスすることができます。
スタディマネージャーを割り当てる
注意!組織管理者のみがスタディマネージャーを追加できます。
スタディマネージャーを追加するには:
| 1 | Viedoc Adminでユーザーを招待する試験を開きます。 |
| 2 | 試験担当者フィールドのツールボックスアイコンをクリックします。 試験担当者のポップアップが開きます。 |
| 3 |
試験ユーザーの追加タブで、招待するユーザーの電子メールアドレスを入力します。次へをクリックします。
|
| 4 |
招待する対象のロールを選択します。+アイコンをクリックすると、複数のロールを追加することができます。新しく追加されたロールは、- アイコンをクリックすると削除することができます。 |
| 5 |
招待を送信をクリックします。 |
組織からユーザーを削除する
Viedocでは、任意のユーザーから組織内のすべての試験における、すべてのロールを一度に削除することが可能です。組織から削除することができるのは、有効になっているロールを持つユーザーのみです。ユーザーに保留中の招待がある場合、そのユーザーを組織から削除することはできません。
組織からユーザーを削除できるのは組織管理者のみです。
注意!この操作を実行しても、ユーザーアカウントは削除されません。削除されるのは、対象ユーザーの組織内のロールと権限のみです。そのユーザーは引き続きログインおよびログアウトすることが可能ですが、その組織の試験を閲覧することはできません。
ユーザーからすべてのロールを一度に削除するには:
| 1 |
ユーザー画面でロールを削除するユーザーを見つけ、ユーザーの名前の横にあるツールボックスアイコンをクリックします。 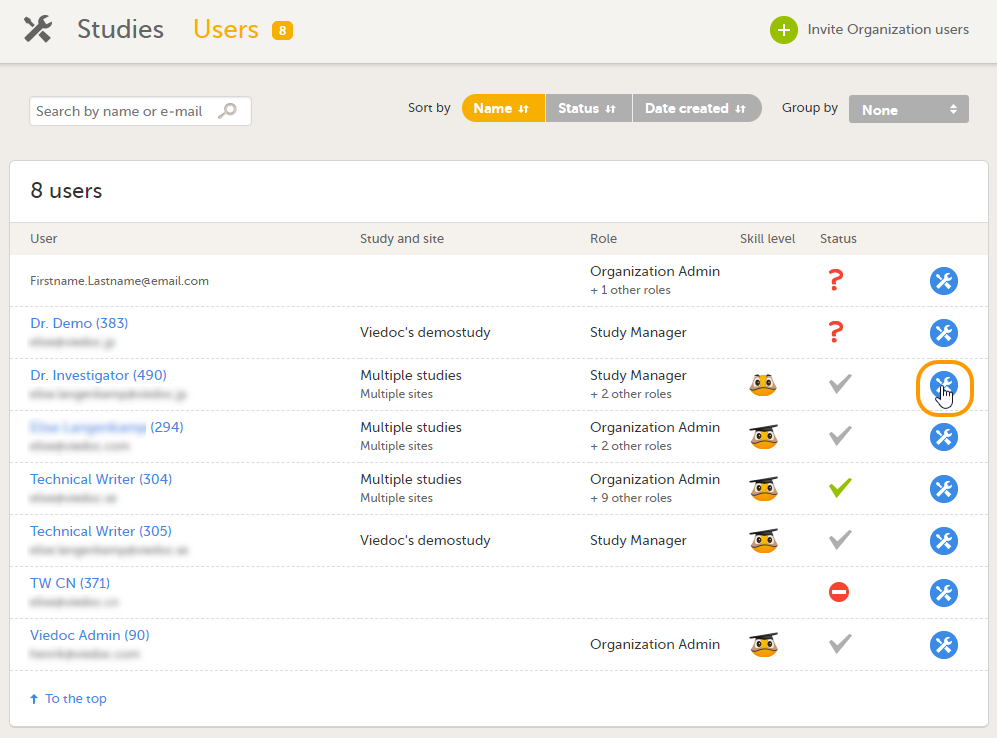
ユーザー設定のポップアップが開きます。 |
| 2 |
組織からユーザーを削除をクリックします。 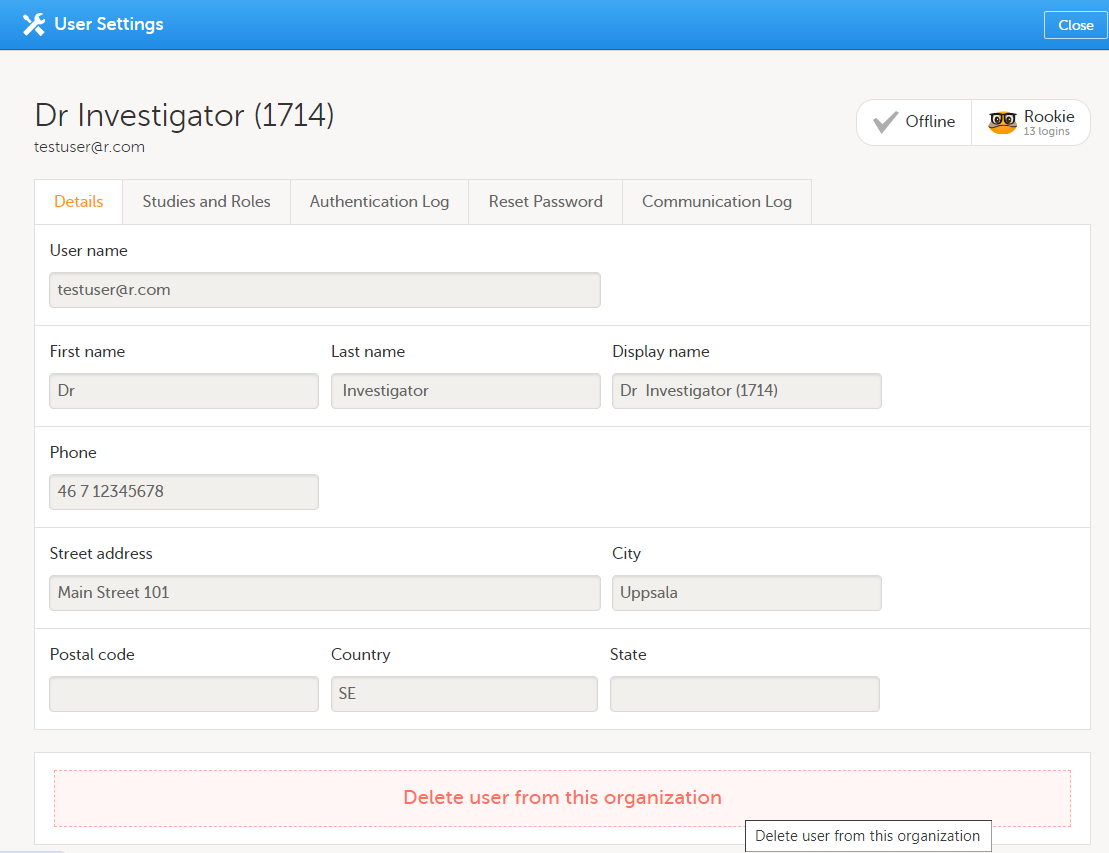
|
| 3 |
削除をクリックして、ロールの削除を確定します。 |
ユーザーロールレポートのダウンロード
ユーザーロールレポートをダウンロードするには:
| 1 |
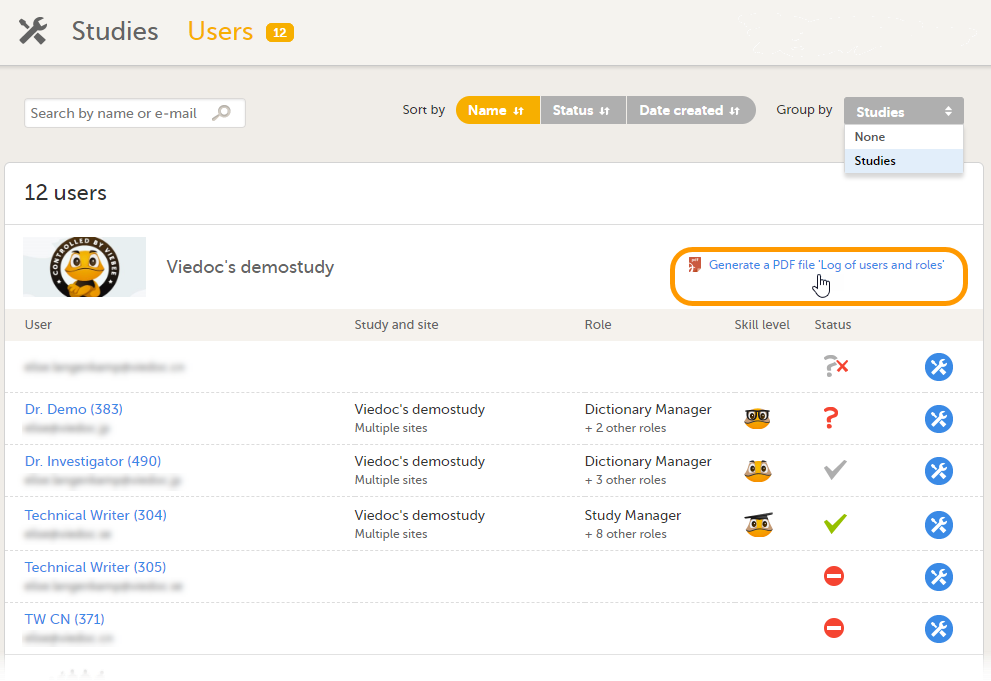
ユーザー画面で、ユーザーを試験でグループ化するように選択します。 |
| 2 |
ユーザーレポートをダウンロードする試験までスクロールします。過去にこの試験のユーザーログのPDFを作成したことがない場合は、ユーザーログのPDFを作成のリンクをクリックすることで作成できます: その結果、すべてのロールとユーザー、権限、サイトごとに並べ替えられたユーザーログ、およびユーザーごとに並べ替えられたすべてのユーザーアカウントログの完全な履歴を含むPDFファイルが作成され、ダウンロード可能になります: このPDFファイルが作成された後、次のいずれかを選択することができます。
または
|

 組織チームのポップアップが開きます。
組織チームのポップアップが開きます。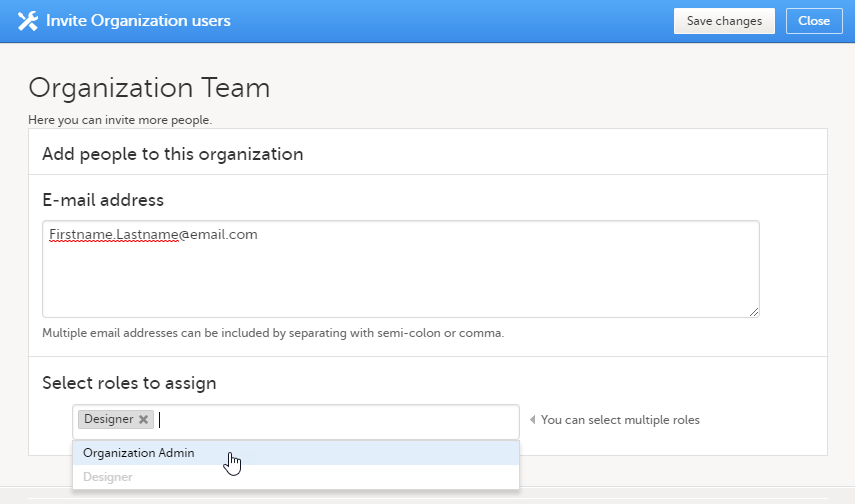
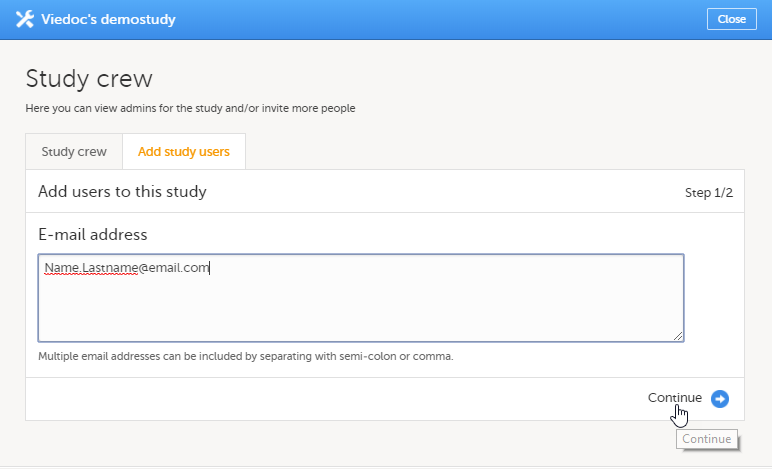 試験ユーザーの追加タブで、招待するユーザーの電子メールアドレスを入力します。続行をクリックします。
試験ユーザーの追加タブで、招待するユーザーの電子メールアドレスを入力します。続行をクリックします。