Importing data from ODM file
Introduction
About ODM import to Viedoc
Viedoc supports the import of data using the Clinical Data Interchange Standards Consortium (CDISC) Operational Data Model (ODM) Extensible Markup Language (XML) standard format, making it possible to migrate data from other Electronic Data Capture (EDC) systems to Viedoc.
ODM is a vendor-neutral, platform-independent format for exchanging and archiving clinical study data. ODM includes all information (clinical data, along with its associated metadata, administrative data, reference data, and audit information) necessary to share data among different software systems during study setup, operation, analysis, and submission. ODM also includes all information for long-term retention as part of an archive to facilitate the regulatory-compliant acquisition, archival and exchange of metadata and data. For more information see https://www.cdisc.org/standards/data-exchange/odm.
In Viedoc Admin, you can import data from another EDC system (including Viedoc 3) using the ODM standard format to Viedoc by uploading an ODM XML file. Viedoc supports data import:
- As per standard CDISC ODM format, or
- Using an ODM file that contains Viedoc extensions (for example, previously exported from Viedoc, see Exporting data). The Viedoc extensions are always prefixed with "
v4:".
Good to know before starting an import
|
Important!
|
Limitations of the ODM import
The following data is not included in the ODM import:
- Medical coding
- Queries
- Review status
- Clinical data history (only snapshot is supported)
How are data mapped during the import of an ODM file?
Import of study sites
The system performs an automatic mapping based on site Name (not case-sensitive). If the Code extension is present (for example if the ODM file originates from Viedoc), this is mapped as well. If this is empty, only the Name is used.
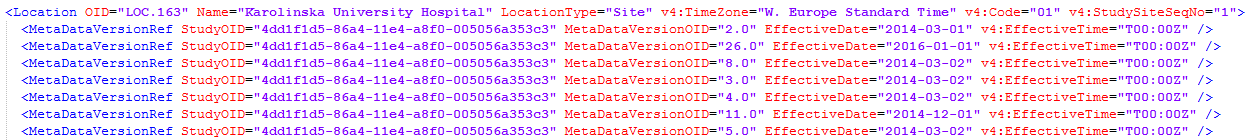
If v4:TimeZone is present in the ODM file, this will be used during the import. If this is not present, the UTC time zone will be used.
If v4:StudySiteSeqNo is present in the ODM file, this will be used during the import. If this is not present, it will be assigned the following value: the maximum v4:StudySiteSeqNo + 1.
If <v4:Address> and <Country> are present in the ODM file, these will be used during the import, otherwise the default will be “SE” (Sweden).
Import of users
The users are imported by full name and email address.
Note! The users are not active immediately after the import is performed. They are only imported to the system. You have to send invitations to these users using the imported email addresses in order for them to get login access to Viedoc.
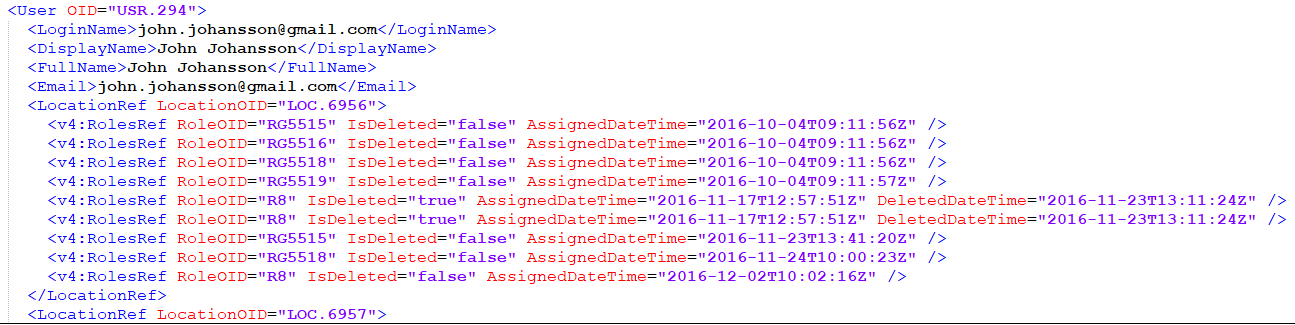
The element LocationRef allows specifying which sites a user is invited to.
The element v4:RolesRef is a Viedoc extension that allows specifying which roles the respective user has for a specific site, as well as the date when the role was assigned/deleted.
Import of subjects
When importing an ODM file to an existing study with existing data, the first step is to assign the subject to a site. This is done by using the subject's SiteRef information in the ODM file:
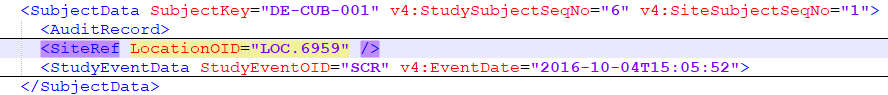
All the sites in the ODM file to be imported (LocationOIDs) are mapped to existing site(s) or new one(s), as described at Step 2/5, prior to the subjects mapping.
A subject is identified in the ODM file by the SubjectKey attribute, which is a standard ODM parameter (string) and it corresponds in Viedoc to the Subject ID that is generated in Viedoc according to the Subject Id Generation Settings.

Mapping to existing subjects by SubjectKey
The subject mapping is performed using the SubjectKey.
- The system checks if any of the existing subjects in Viedoc, within the specified site, has the Subject ID identical with the provided
SubjectKey, and:
- If a match is found, then the data is imported to the existing subject.
- If no match is found then a new subject is created within the specified site and the data is imported to it.
- After the data is imported, the Subject ID is generated according to the Subject Id Generation Settings and using the newly imported data.
When a new subject is created in Viedoc, there are two behind-the-scenes system items created for it:

v4:StudySubjectSeqNois a sequence number of subjects on study level. If a subject is the second subject in the study, this item is 2.v4:SiteSubjectSeqNois a sequence number of subjects on site level. So if the same subject is the first subject on the site, this item is 1.
These two system items (Viedoc extensions) are used for various purposes, of which the Subject ID is the most important, see Subject Id Generation Settings. For a newly created subject, these sequence numbers can be:
- either parsed from the provided
SubjectKey, if they are being used in the Subject Id Generation Settings for the study the data is imported to, or otherwise - allocated the next available sequence numbers within the study (
v4:StudySubjectSeqNo) and site (v4:SiteSubjectSeqNo) respectively.
Notes!
- If the ODM file to be imported originates from Viedoc and it was exported including the Viedoc extensions, these sequence numbers are included in the ODM file.
- If any of the
v4:StudySubjectSeqNoorv4:SiteSubjectSeqNois either provided in the ODM file to be imported or mapped during the import process (at Step 3/5 described later on), these are used to perform the subject mapping, see Mapping to existing subjects by StudySubjectSeqNo and/or SiteSubjectSeqNo below.
Mapping to existing subjects by v4:StudySubjectSeqNo and/or v4:SiteSubjectSeqNo
These two system items (Viedoc extensions) are used for various purposes, of which the Subject ID is the most important, see Subject Id Generation Settings.
If any of these sequence numbers is provided in the ODM file as Viedoc extension, or if they are mapped during the import process (see Step 3/5 below), the subject mapping is performed as follows:
- If any of the sequence numbers is provided, these are used to perform the matching, first by the
v4:SiteSubjectSeqNoand then byv4:StudySubjectSeqNo. - If the sequence numbers are provided, but no match is found, then the
SubjectKeyis used for mapping, as described above in Mapping to existing subjects by SubjectKey.
Note! If the sequence numbers for these items are present in the ODM file, but they are also mapped during the import process (see Step 3/5 below), then the mapping takes precedence.
Workflow
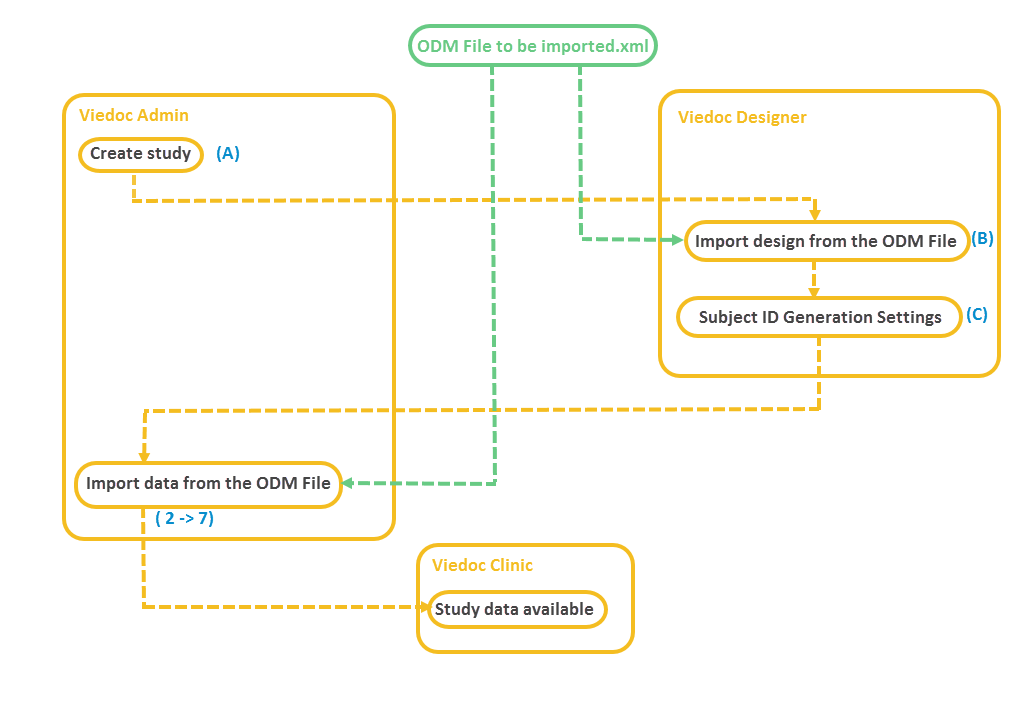
Before you start importing the ODM file, you have to make sure that you already have a study in Viedoc that has a study design that matches the data structure in the ODM file to be imported. The metadata version(s) in the ODM file to be imported must contain all the events, forms, item groups, items and code list values that are referenced by ClinicalData. The import process performs the matching only by using OIDs.
In case you do not have such a study yet, you can create a study and perform the ODM import as described below:
A. Create a study in Viedoc Admin (for instructions see Adding new study) and invite a user as Study Designer. This user will get access to Viedoc Designer.
B. In Viedoc Designer, import the design from the ODM file to the study you have just created in Viedoc Admin (for instructions see Initiating a design).
C. In Viedoc Designer, open the study with the newly imported study design, go to Study Settings and configure the Subject ID Generation Settings (for instructions see Subject Id Generation Settings). This will impact the selection you have to make later on during the import in Step 3/5.
Note! Step C does not have to be performed if the ODM file has been exported from Viedoc 4 including extensions.
Importing an ODM file
This section provides a step by step guide for importing an ODM file.
Step 1/5 - uploading the ODM file
In Viedoc Admin, go to the study into which the data should be imported. Click Study Settings.
The Study settings pop-up opens.
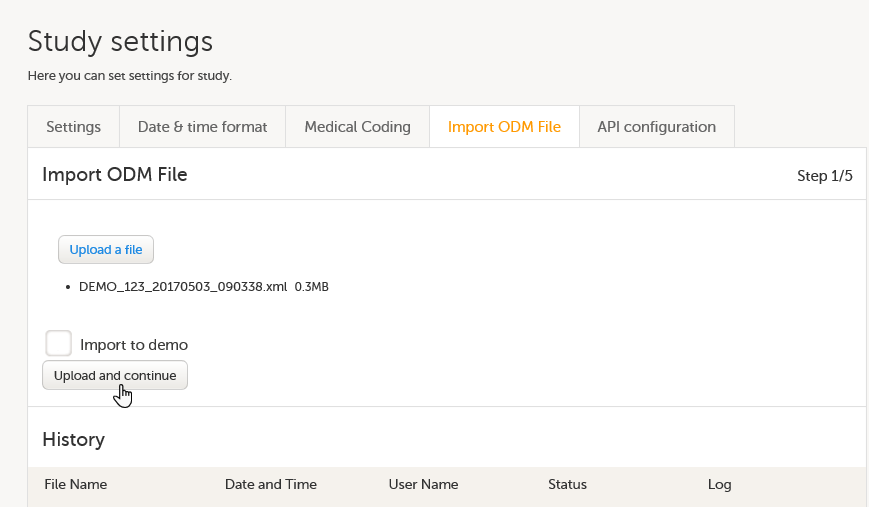 On the Import ODM File tab, click Upload a file, and browse to the ODM file you would like to import. The file name and size will appear right under the Upload a file button.
On the Import ODM File tab, click Upload a file, and browse to the ODM file you would like to import. The file name and size will appear right under the Upload a file button.
If you would like to import the ODM file to a demo version of the study, select the Import to demo checkbox.
In case you receive an error message saying that the file cannot be uploaded due to missing content (according to the CDISC ODM standard), you have to go back to your ODM file, fix the error and upload the file again.
Click Upload and continue. This takes you to step 2/5.
Step 2/5 - mapping the study sites
In the Metadata version to study design version mappings field, two columns are displayed. Metadata version OID (from xml) lists all the versions found in the ODM file you have uploaded. In the Study design version select the study design version in Viedoc that the data should be imported into. The design has to match exactly your ODM data to be imported.
In the Study site mappings field, three columns are displayed. Column 1 and 2 (see image) represent all the sites found in the ODM file you have uploaded. Column 3 represents the sites available in the study you have selected to import into. The system performs an automatic mapping based on site name (not case sensitive). If the code extension is present (if the ODM file originates from Viedoc), this is mapped as well. If the code extension is empty, only the name is mapped. If no match is found, the system will map to “Create new site” as a default.
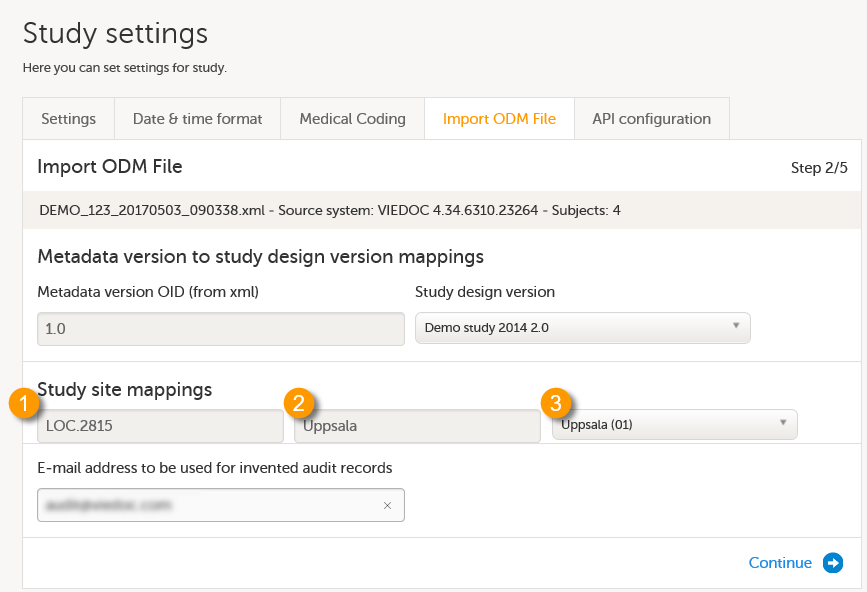 Check whether the automatic mapping performed by the system is correct. If necessary, manually perform the mapping by selecting a site from the drop-down list.
Check whether the automatic mapping performed by the system is correct. If necessary, manually perform the mapping by selecting a site from the drop-down list.
Note! If a match is found but you anyway select Create new site from the drop-down list, a duplicate site will be created. This is not recommended!
Note! Make sure that every Location in the ODM file to be imported has at least one MetaDataVersionRef defined, otherwise no design version will be assigned to the respective site.
In the Email address to be used for invented audit records field, enter an email address that can be used when the import needs to create audit records.
Click Continue. This takes you to step 3/5.
Step 3/5 - mapping the study event dates
Under Study event dates, select what date items you want to be matched to your events. If no selection is made and a form and item combination within the event called $EVENT.EventDate is found (the way Viedoc stores event dates), this will be used. If the ODM file originates from Viedoc 4, and has been exported including extensions, you will find this form/item combination in the drop-down list.
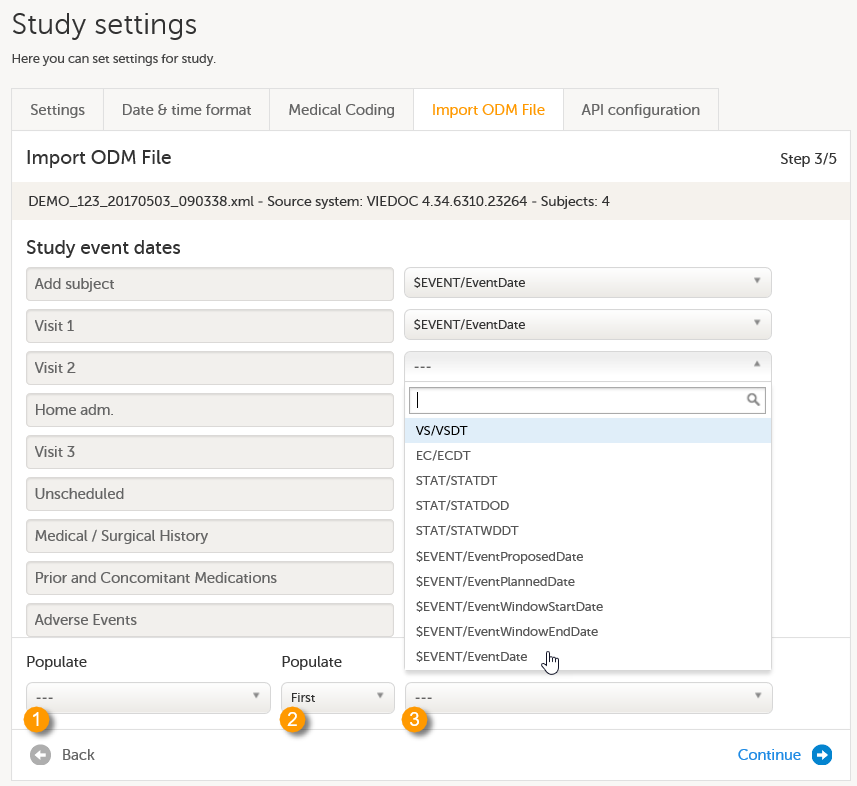
The settings to be performed under Populate depend on whether the ODM file to be imported originates from Viedoc and thus has the SiteSubjectSeqNo and/or StudySubjectSeqNo extensions, or not. See also Import of subjects.
- If the ODM file has the SiteSubjectSeqNo and/or StudySubjectSeqNo extensions, then no settings are required at this step. These items will be imported automatically. You can continue to Step 4/5.
- If the ODM file does not have the SiteSubjectSeqNo and/or StudySubjectSeqNo extensions, the settings to be made depend on how the Subject ID Generation Settings are configured in Designer (see Workflow).
- If you have used one of the SiteSubjectSeqNo or StudySubjectSeqNo items, select this item from the first Populate drop-down list (1 in the image), and then select the form this item will be picked from in the (Form/Item) drop-down list (3 in the image). If the item you selected occurs multiple times for a single subject, you can select whether the first or last occurrence should be used in the second Populate drop-down list (2 in the image, default is First).
- If you have used a different variable than the SiteSubjectSeqNo and StudySubjectSeqNo, then you don't need to make any selection in the Populate fields.
Note! Once you have selected an option from the drop-down list, it is not possible to clear the selection and return to the default (--- or no selection). It is only possible to select another option from the drop-down list.
Click Continue. This takes you to step 4/5.
Step 4/5 - selecting events and forms to be excluded
In the Select events to be excluded field, click and select from the drop-down list the events that you do not want to be included in the imported study. If you want to exclude multiple events, click and select again.
In the Select forms to be excluded field, click and select from the drop-down list the forms that you do not want to be included in the imported study. If you want to exclude multiple forms, click and select again.
| Important! When importing an ODM file that was exported from Viedoc 4, you must exclude the $EVENT form. |
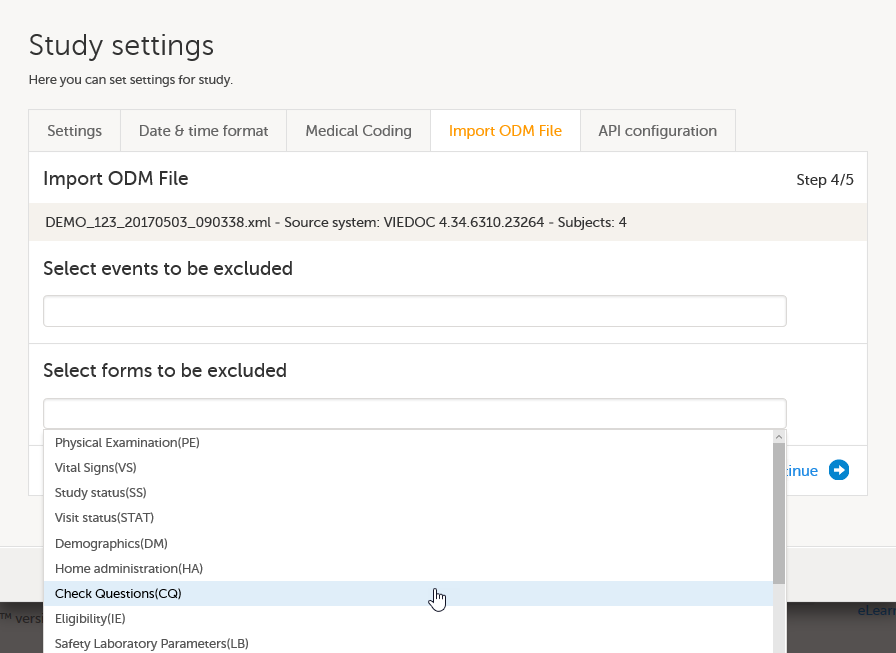
Click Continue. This takes you to step 5/5.
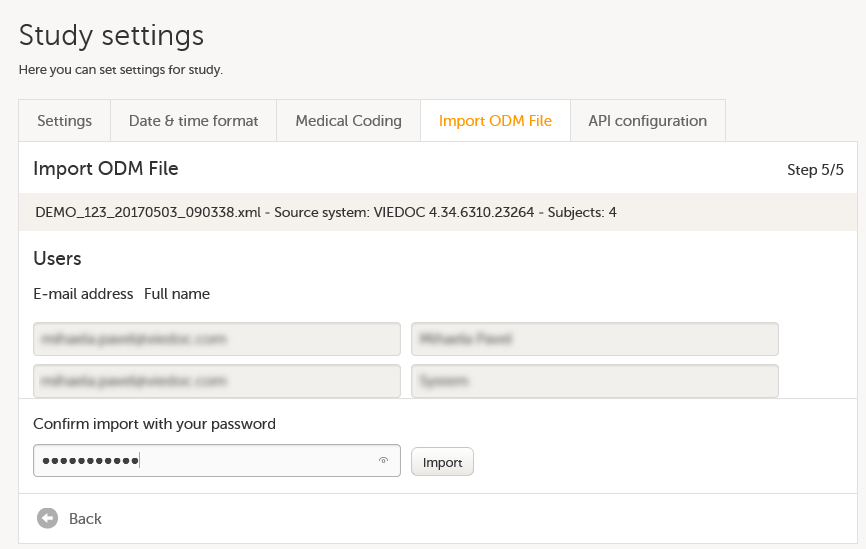
Step 5/5 - confirming the import
In the Users field, a list of the imported users identified by email address and full name is displayed. In the Confirm import with your password field, enter your password to confirm the list of users to be added to your study, and click Import.
Note! The users are not active immediately after the import is performed. They are only imported to the system. You have to send invitations to these users using the imported email addresses in order for them to get login access to Viedoc. For instructions see Managing users.
After the import
Once you have imported the file and invited the users, your study is available in Viedoc and accessible for the users you have invited.
The PDFs with form history are not immediately available after the import. They will be generated and become available in Viedoc after you have performed an export to PDF in Viedoc Clinic. For instructions, see Exporting data.
All the functions are re-executed after the import.
