Overview of Viedoc eTMF
Overview
Viedoc eTMF is a digital repository for capturing, managing, sharing, and storing essential documents for your clinical trial.
Viedoc TMF is based on the TMF Reference Model by the Drug Information Association (DIA). The TMF Reference Model is an industry consensus catalog of all TMF records. Using the TMF Reference Model ensures compatibility and interoperability with other clinical trial parties, such as CROs.
The TMF Reference Model includes documents in all different phases of a clinical trial:
- Before the start of the trial
- During the trial
- After study termination
The TMF Reference Model categorizes documents in zones, sections, and artifacts in a hierarchical structure.
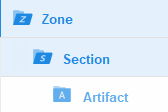
The set of zones, sections, and artifacts included is defined in a template file that is maintained by the eTMF Manager.
The TMF can include both the Investigator Site File (ISF) and the sponsor TMF.
For portability reasons, the DIA TMF Reference Model is defined in an Excel file.
Viedoc eTMF also uses Excel files as templates for the eTMF structure.
User roles
In Viedoc Admin, the Study Manager assigns an eTMF Manager. For more information, see Managing users.
The eTMF Manager can then map Viedoc user roles to eTMF user roles and permissions. For more information, see Managing your Viedoc eTMF application.
Document statuses and actions
The following image shows the document version statuses and the actions that change the status of a document version. The initial status of a document when it is uploaded to Viedoc TMF is Unpublished.

If you edit the metadata for a document version that is Unpublished or Awaiting review, the document version status is not changed.
It is not possible to edit the metadata of a Finalized document. To make changes, a new version needs to be created.
Note! Different actions require different permissions, which means that they are performed by users with different roles.
Permissions
The Viedoc eTMF Manager permissions
The eTMF Manager has permissions to manage the eTMF application in Viedoc Admin and to manage templates in Viedoc eTMF.
Viedoc TMF end user roles and permissions
The user access to Viedoc TMF is determined by the assigned roles and permissions. The roles and permissions can work in combination or independently.
eTMF user roles
These user roles are defined in the template, which is maintained by the eTMF Manager.
The end user role determines what kind of access a user has to artifact data (no access/read/write/review) on the different levels:
- Study/trial
- Country
- Site
eTMF permissions
Note that this text section is available also in the lesson Introduction to Viedoc eTMF.
These permissions are defined in Viedoc and are assigned to the users by the eTMF Manager.
The eTMF permissions are:
- Archive sponsor TMF
This permission gives the mapped user role access to the TMF Archive view and the ability to archive artifacts that are listed as Sponsor side (set in the Edit artifact window or in the template file on the sheet V 3.1.0, column M Sponsor Document). This is used for creating the main archive of the study documents. - Archive investigator TMF
This permission gives the mapped user role access to the TMF Archive view and the ability to archive artifacts that are listed as Investigator side (set in the Edit artifact window or in the template file on the sheet V 3.1.0, column N Investigator Document). This is used for creating/archiving an Investigator Site File. - Read-only TMF Admin
This permission gives the mapped user role the ability to inspect the structure, templates, and other settings in the TMF Admin view in read-only mode.
A user with this permission can access the TMF Admin view and is able to:- View a selected/instantiated structure
- Export templates and structure
- View the settings tab
- Read-only Trial Master File
A user role with this permission will gain read access to all the published documents in the Trial Master File view. If this permission is assigned in combination with an eTMF role, the no access permission, set in the template file for that specific role, will be overridden by read access by the system. - Download audit trail
A user role with this permission will be able to access the TMF Archive view and generate the complete audit trail report from there. - Manage drop zone
This permission gives the mapped user role access to manage the files in the shared drop zone. - Manage document sharing for Viedoc Clinic users
A user role with this permission can share documents with Viedoc Clinic users. - Manage document sharing for Viedoc Me users
A user role with this permission can share documents with Viedoc Me users.
Note! For more information about permissions and accesses, see eTMF access use cases.
Viedoc TMF views
TMF views for the eTMF Manager
If you have the user role eTMF Manager or the permission Read-only TMF Admin, you have access to the TMF Admin view. In this view, the eTMF Manager can manage the eTMF templates, structure, and other eTMF settings.
If you have the user role eTMF Manager, a Viedoc Clinic role that is mapped to an eTMF role, and have the permission to Download audit trail report, you have access to three views of Viedoc eTMF: TMF Admin, Trial Master File, and TMF Archive.
- The TMF Admin view is where you manage the eTMF structure.
- The Trial Master File view is where you can access Viedoc eTMF as an end user.
- The TMF Archive view is where you can generate the complete audit trail report.
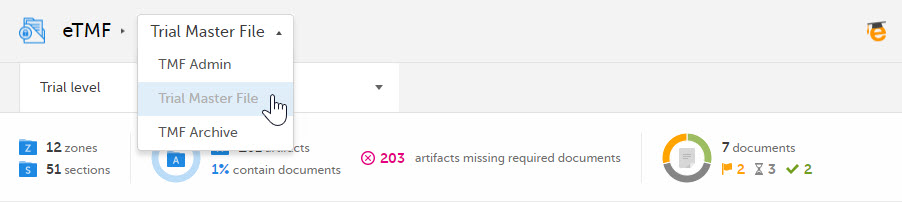
To toggle between the views, use the dropdown menu:
TMF view for end users
See Introduction to Viedoc eTMF.
TMF view for Archivers
See Complete audit trail report.
eLearning
In Viedoc eTMF, there is a link to the eLearning curriculum Viedoc User Guide for eTMF Managers.