TMF Archive
Note! This page contains information and instructions for the new TMF user interface. If you are using the old interface, please go to the relevant user guides:
- Viedoc eTMF User Guide (old interface)
- Viedoc User Guide for eTMF Managers (old interface)
Want to browse more information for the new interface? Please go to the new TMF user guides:
TMF Archive
Users with the appropriate permissions for managing the TMF Archive area of Viedoc TMF will see the following in the left navigation menu:
The next sections describe how to generate the Audit trail report and the eTMF-EMS repository.
Naming conventions
It is best practice to use the record name templates in TMF settings as naming conventions, to ensure all data that you want to appear are included in file names in the archive and repository.
If an archive template name is defined in TMF settings, when archiving, the latest template will be applied to the files in the zipped folder.
By default, if a record name template is not defined for the archive template:
- The filename in the zipped folder will be the same as the latest record name set in record properties
- All files in the zipped folder will have the version of the file as a suffix.
- If there are multiple records with the same name in the same folder in the archive, they will be indicated with -n so that none of them will be overwritten.
- Files in the zipped folder will never be overwritten.
- If a system variable used in the template doesn't exist for a record, that variable will be empty in the template.
- When there is an ongoing archive operation, if the eTMF Manager defines a new archive template, this change will not affect the ongoing archive operations, it will only affect the upcoming archives.
Audit trail report
Introduction
A complete audit trail report can be generated and downloaded in Excel format. It includes a complete list of actions done on:
- the structure, templates, and settings by the TMF Managers
- the records included in the TMF by the users
- generating and downloading the complete audit trail report and the eTMF-EMS repository
The report respects the user roles and access to records, sites, and countries.
Generating and downloading the report
In the left navigation menu, select to expand TMF Archive and select the Audit trail report page.
To generate the complete audit trail report:
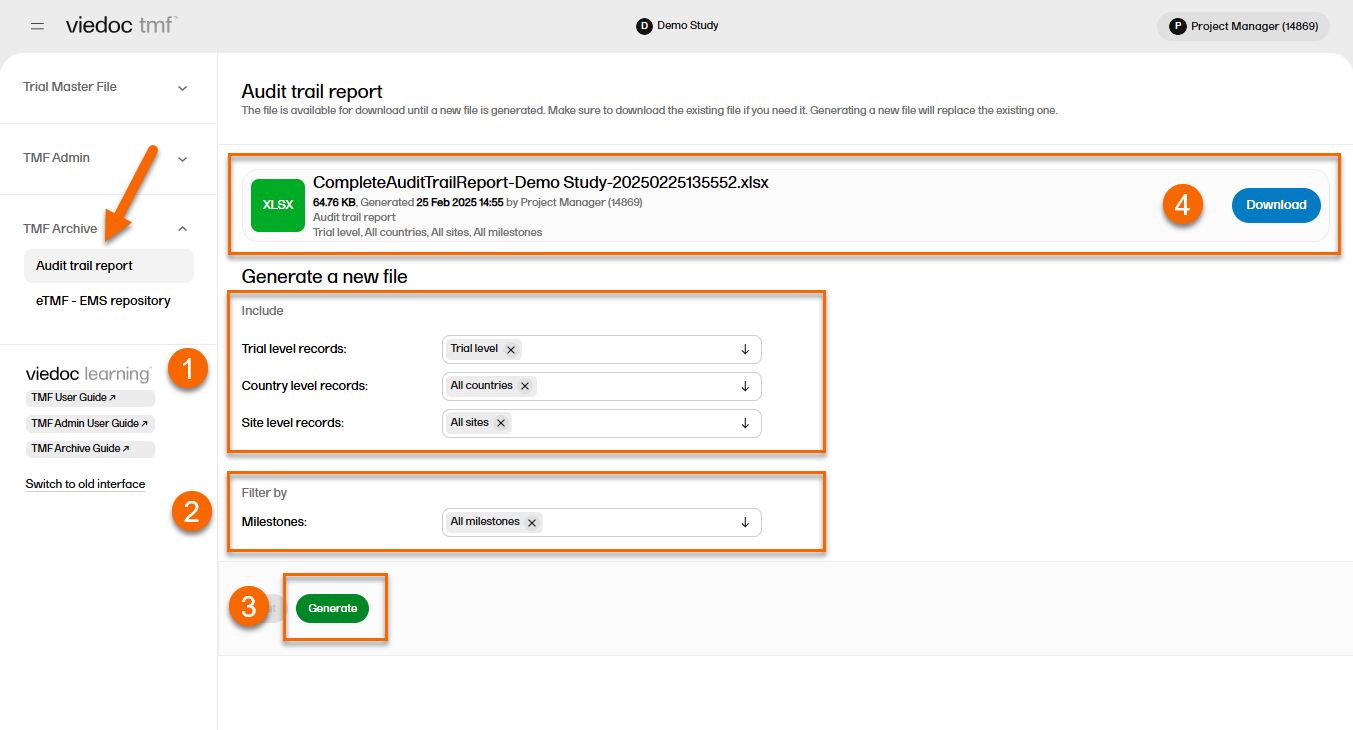
| 1 | Set the options for the records’ audit trails to be included. You can choose to include records that are filed on trial/country/site levels according to your permissions to those. |
| 2 |
Set the milestones/milestone groups you would like to filter by. |
| 3 | Select Generate. |
| 4 | Select Download on the generated file to download the report. |
Included sheets
Each sheet in the complete audit trail report corresponds to the actions done by users in the TMF. The report is self-explanatory, but in the following sections you can find detailed information about each sheet in the file:
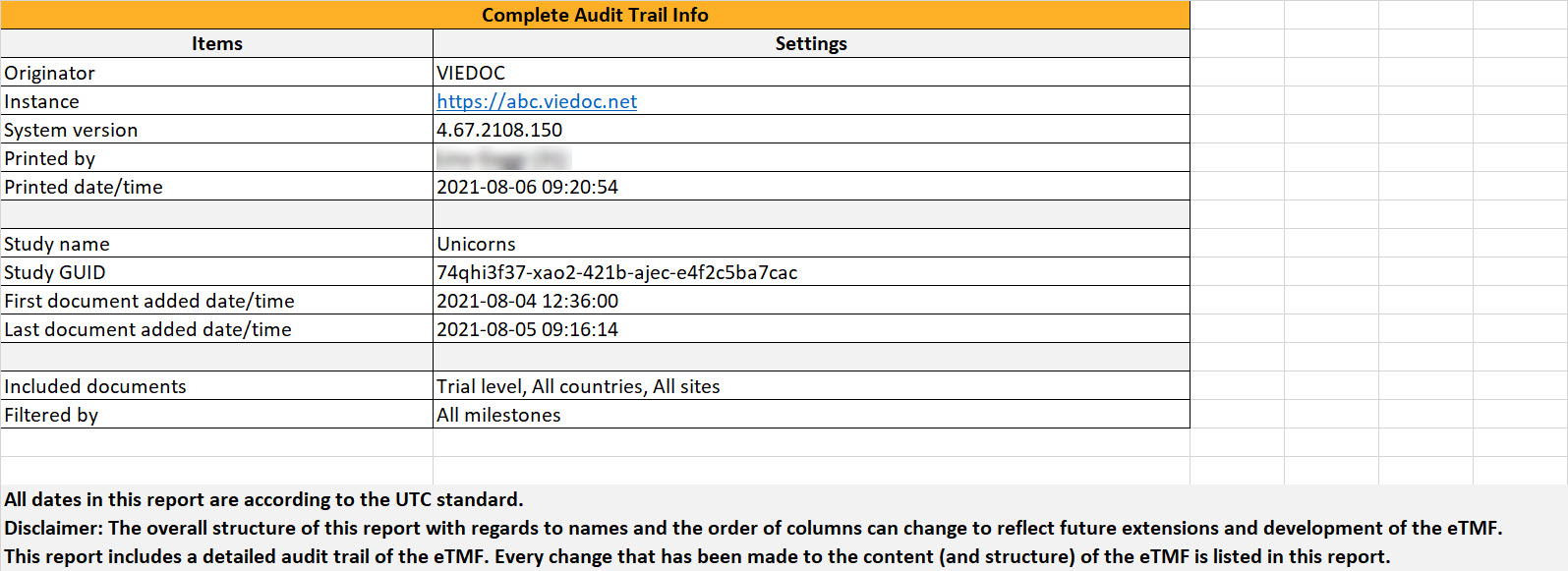
Report Info
This sheet includes general information about the report and the study. The First document added date/time and Last document added date/time show when the first and last records were uploaded to the TMF. This is shown regardless of whether the audit trail of these records is included or not in the report.
Trial Master File
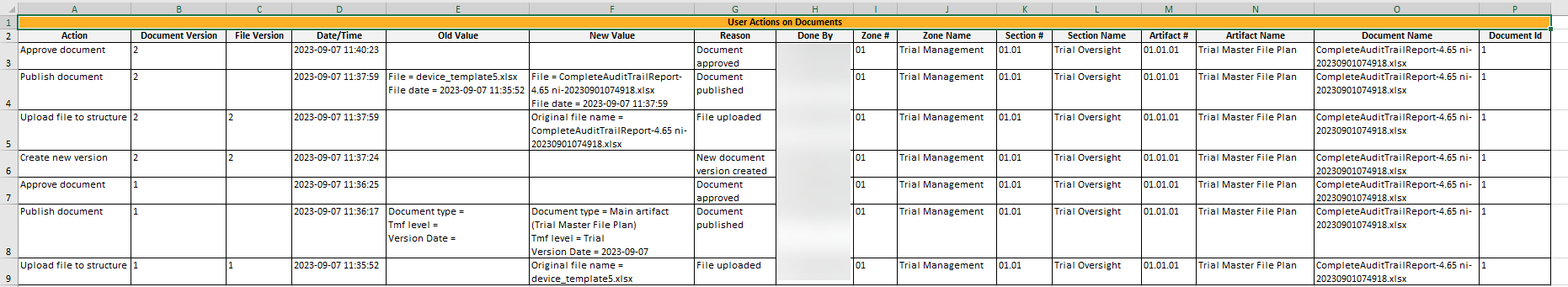
This sheet includes information about all the actions done by users on records in the Trial Master File view. Note that the actions that are included in this sheet are done on:
- Published records only.
- records that are linked to a level (Trial/Country/Site) that the user chose to include and are filed to an artifact that is linked to a milestone that the user has filtered by.
- records that the user has at least READ access to.
Sharing
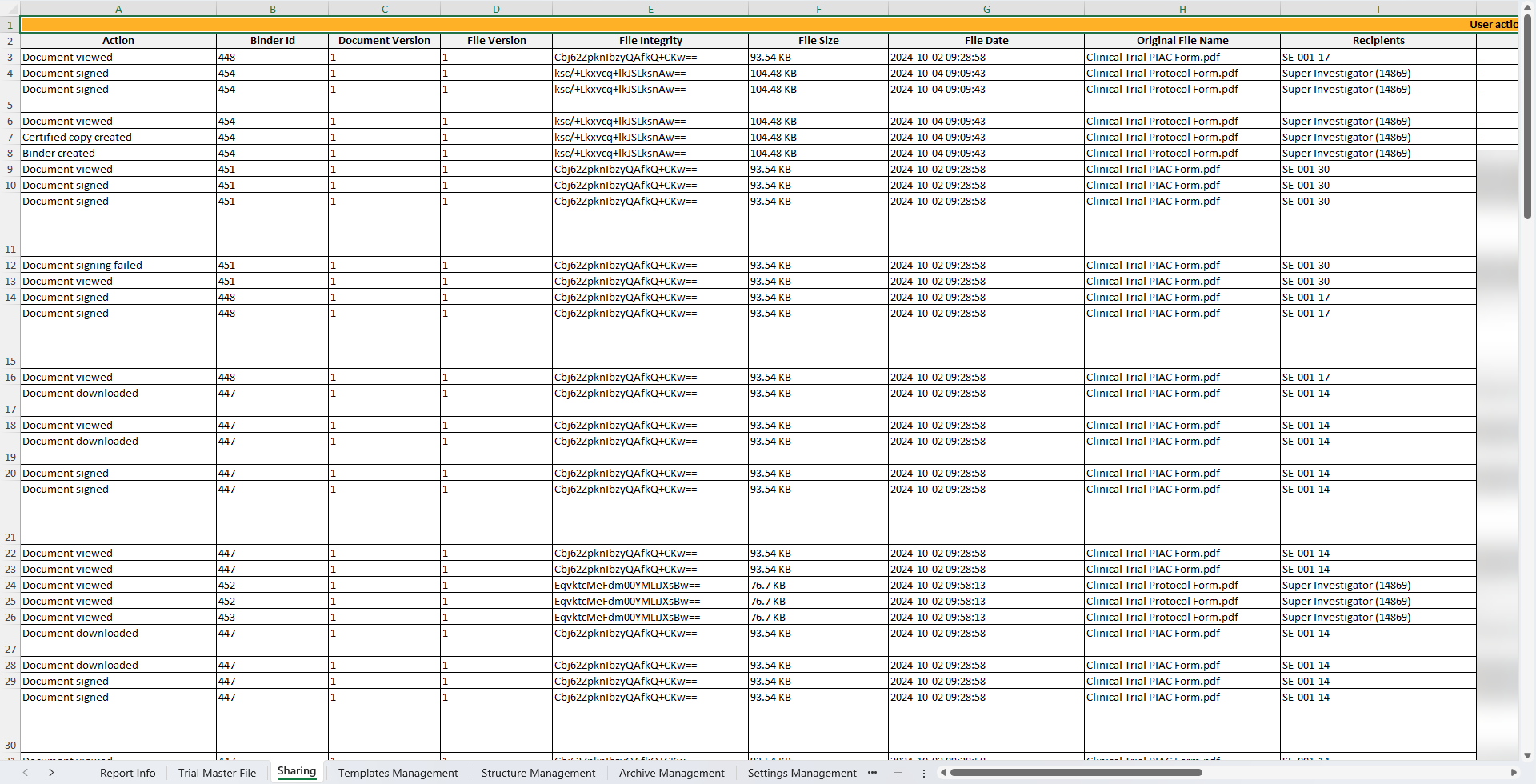
This sheet includes information about all user actions on binders in Viedoc Share and Viedoc TMF.
Note! The sheet only include actions on the binders that the archivist has access to.
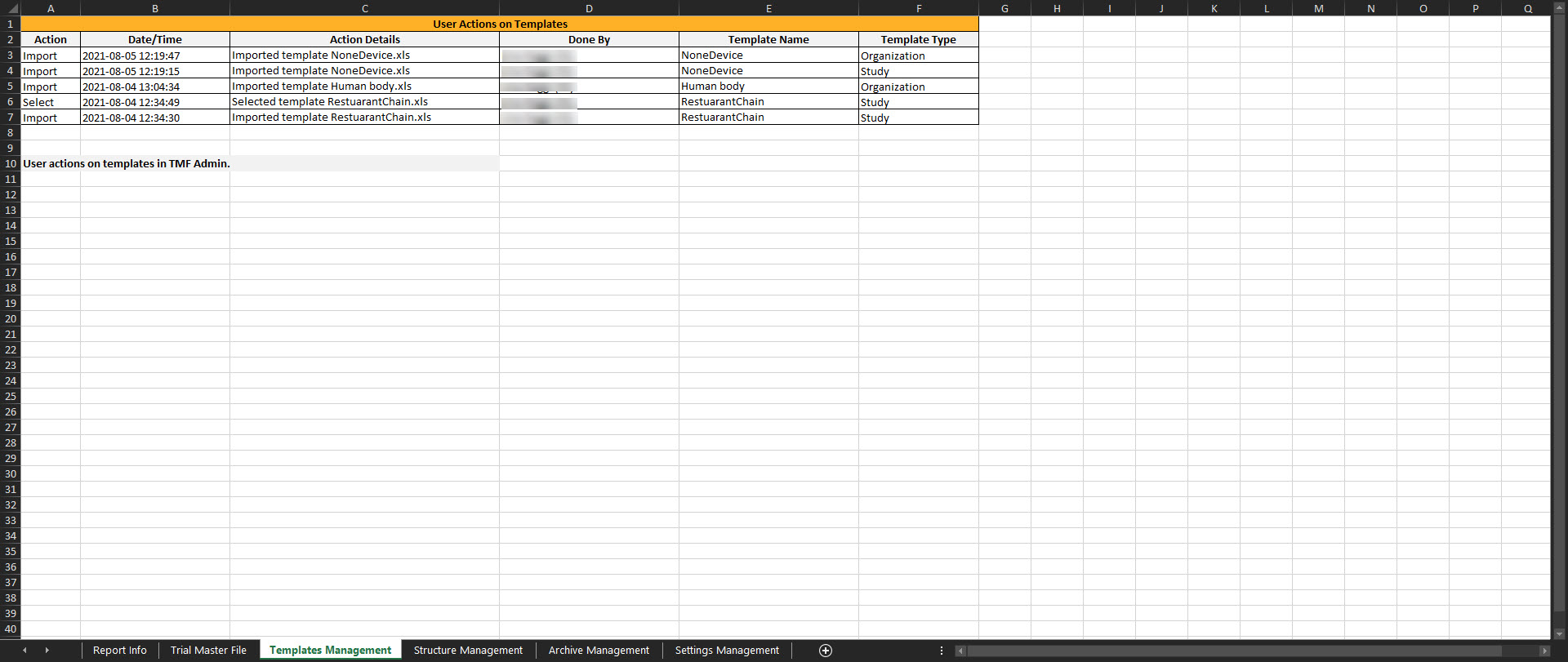
Templates Management
This sheet includes actions done by eTMF Managers on the templates. If the user doesn’t have access to the TMF Admin view, this sheet is empty.
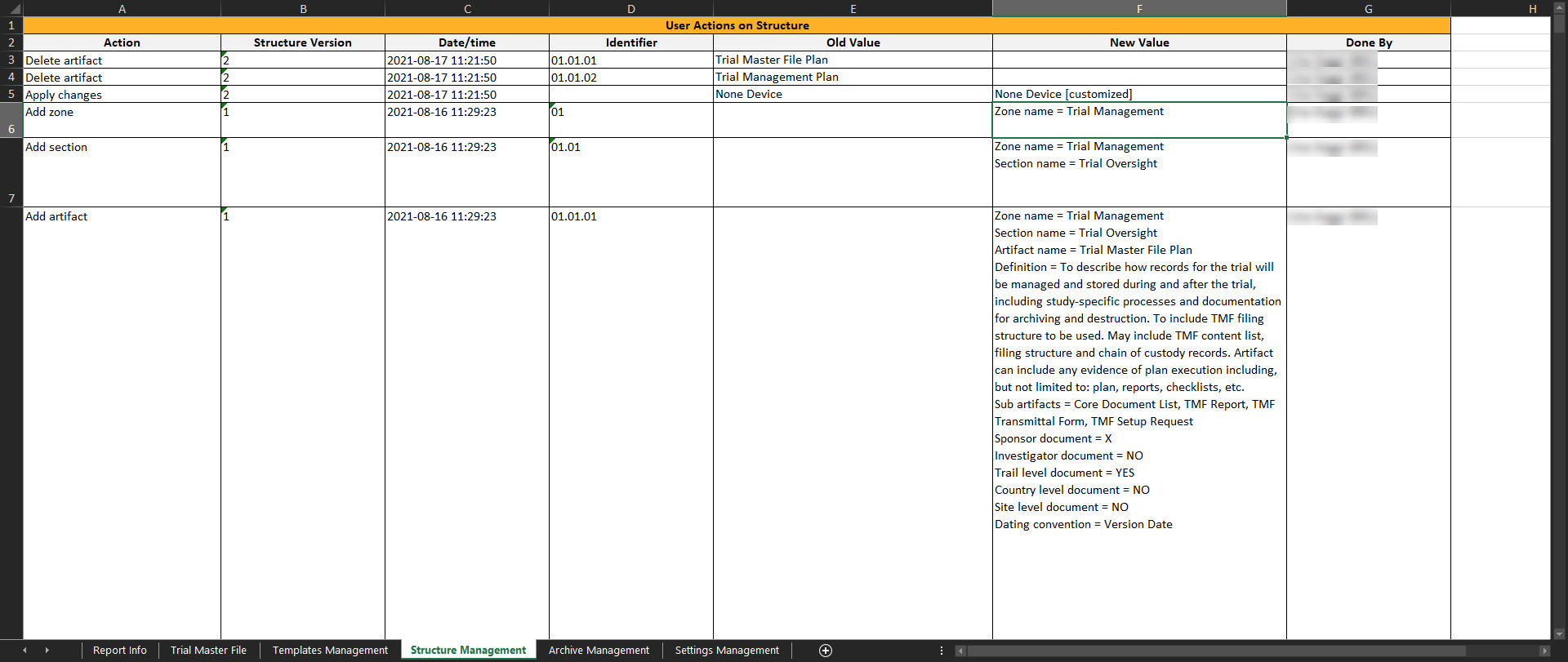
Structure Management
This sheet includes actions done by eTMF Managers on the instantiated structure. If the user doesn’t have access to TMF Admin, this sheet is empty.
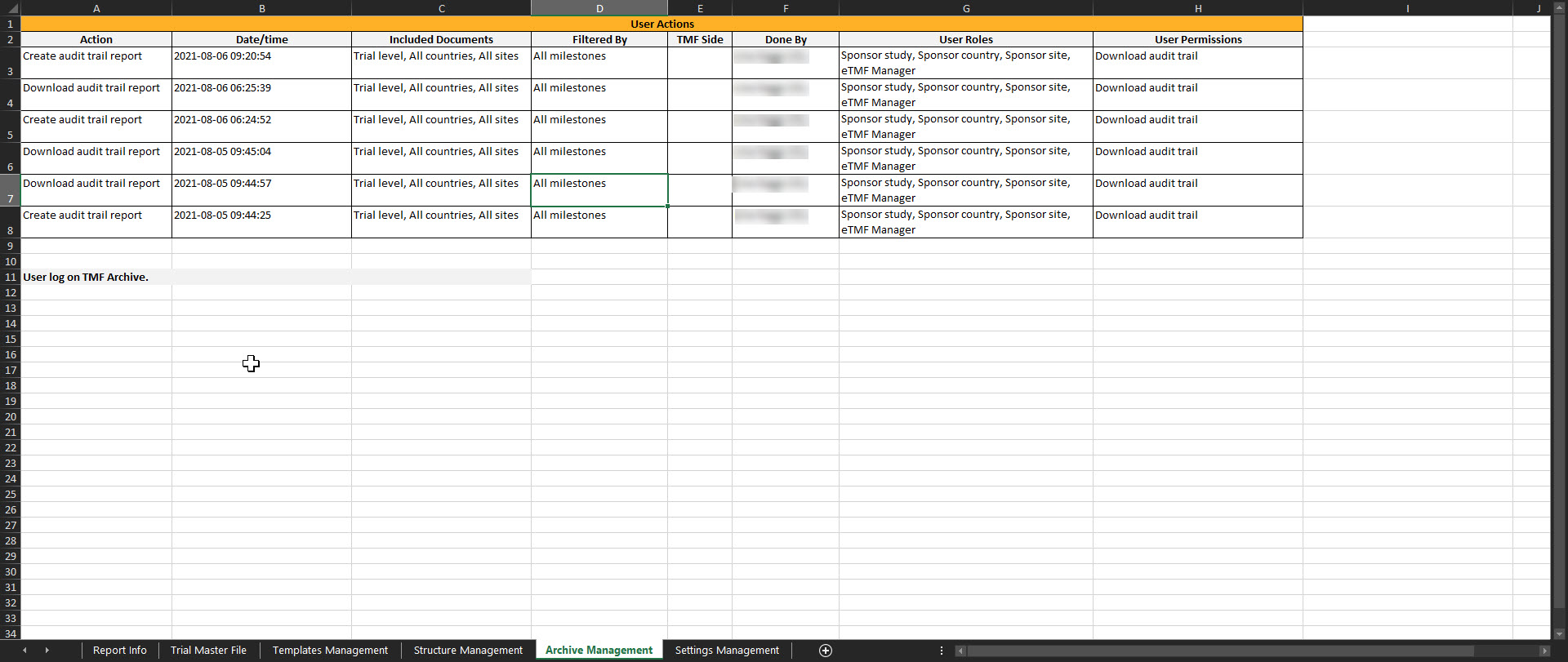
Archive Management
This sheet includes this user actions done on the TMF Archive page.
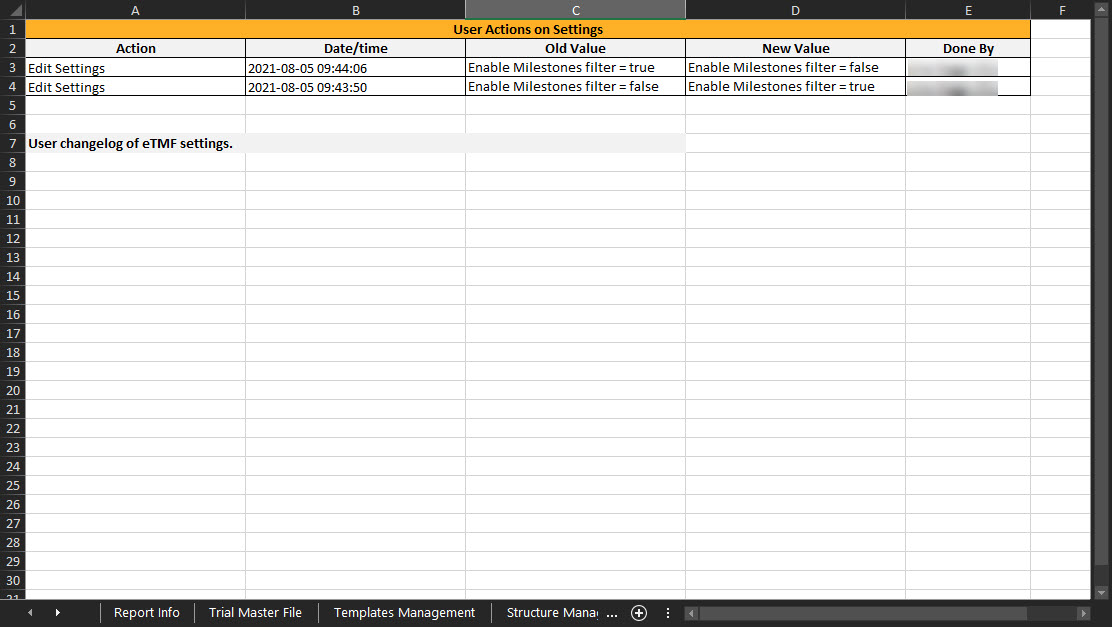
Settings Management
This sheet includes the actions done by TMF Managers on the Settings tab in TMF Admin. If the user doesn’t have access to TMF Admin, this sheet is empty.
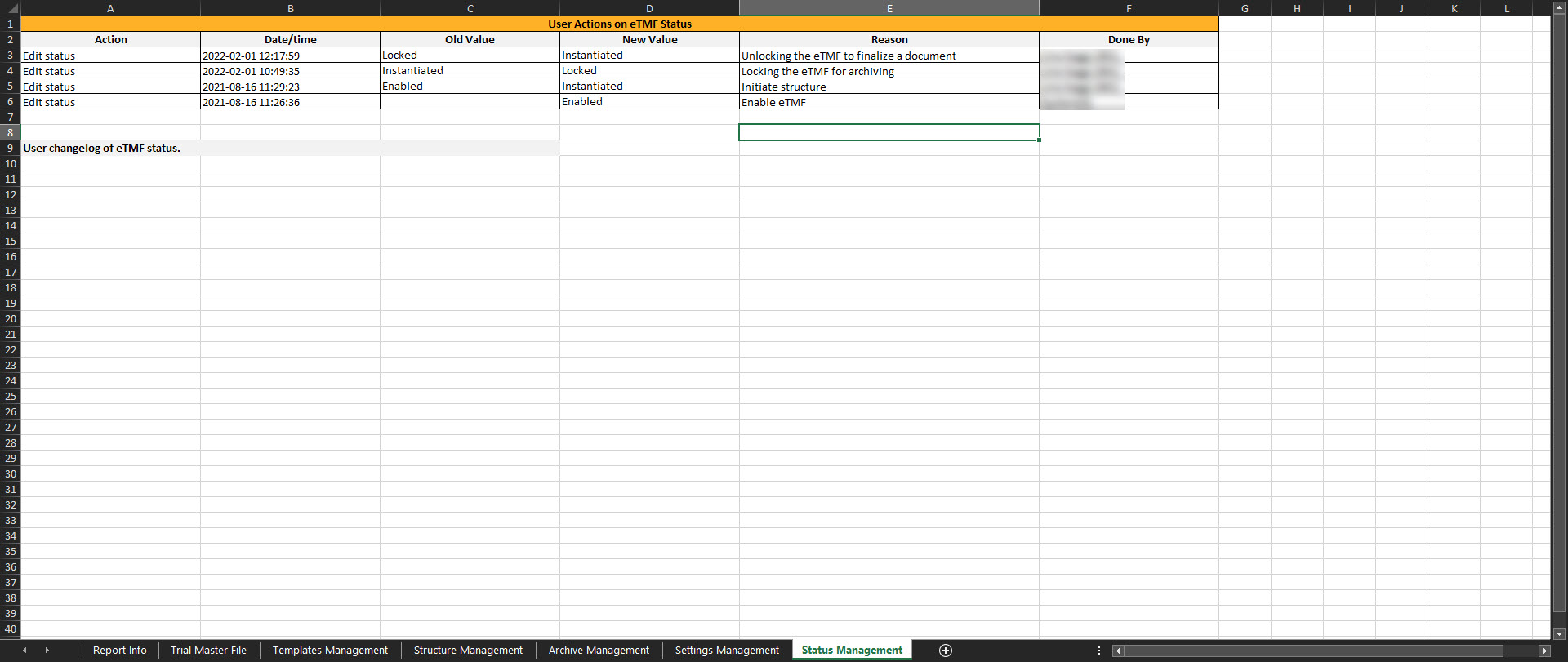
Status management
This sheet includes the actions done by TMF Managers on the Status tab in TMF Admin. If the user doesn't have access to TMF Admin, this sheet is empty.
eTMF-EMS repository
Introduction
The eTMF-EMS repository can be used for archiving the sponsor and/or investigator side of the study and/or exporting the records that are included in the structure. It is compatible with the Exchange Mechanism Standard (EMS). Read more about the EMS here.
The eTMF-EMS repository respects the user roles and access to records, sites, countries, and TMF side.
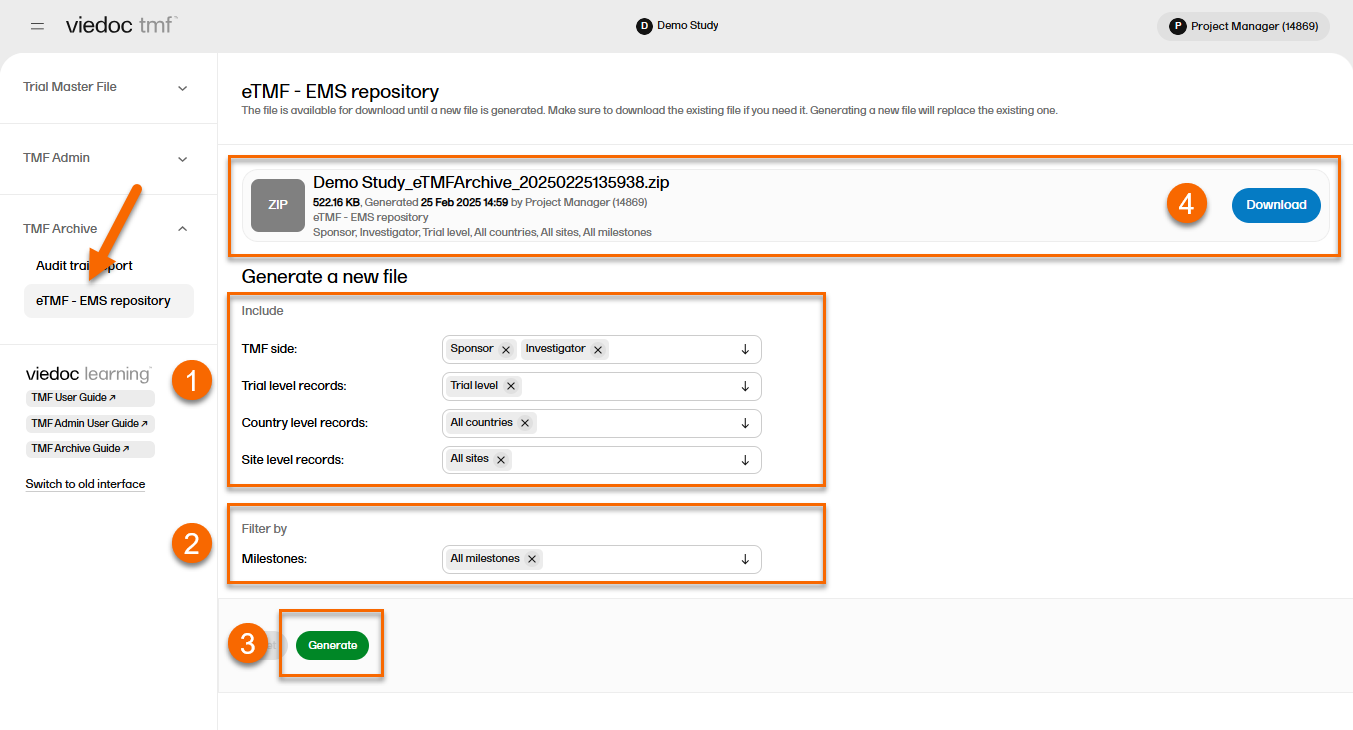
Generating and downloading the EMS repository
In the left navigation menu, select to expand TMF Archive and select the eTMF-EMS Repository page.
To generate the repository:
| 1 | Set the options for the records you want to include in the archive. You can choose to include records that are filed to the investigator or sponsor side of the TMF, and records that are filed on trial/country/site levels according to your permissions to those. |
| 2 | Set the milestones/milestone groups you would like to filter by. |
| 3 | Select Generate. |
| 4 |
Select Download on the generated file to download the zipped folder. |
The zipped folder structure and content
The zipped folder structure mirrors the TMF structure used for the study as follows:
- Level 1 – this level includes:
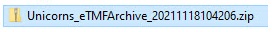
- The main zipped folder with a name in the format:
StudyName_eTMFArchive_DatetimeStampStudyName- the study nameeTMFArchive- static textDatetimeStamp- the UTC date and time of generating the eTMF-EMS repository in the formatYYYYmmDDHHMMss
- The main zipped folder with a name in the format:
- Level 2 – this level includes:
- A subfolder that has the name TransferID (datetime stamp of generating the eTMF-EMS repository) in the format
YYYYmmDDHHMMss - An exchange.xml file for each chosen milestone. You can read about the content of the xml files and find an example here.
- A subfolder that has the name TransferID (datetime stamp of generating the eTMF-EMS repository) in the format
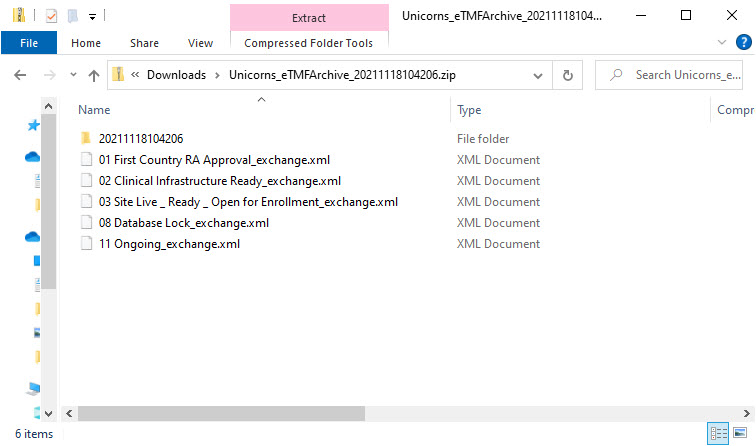
- Level 3 – this level includes:
- A folder with
ZoneID.ZoneNamefor each included zone from the structure
- A folder with
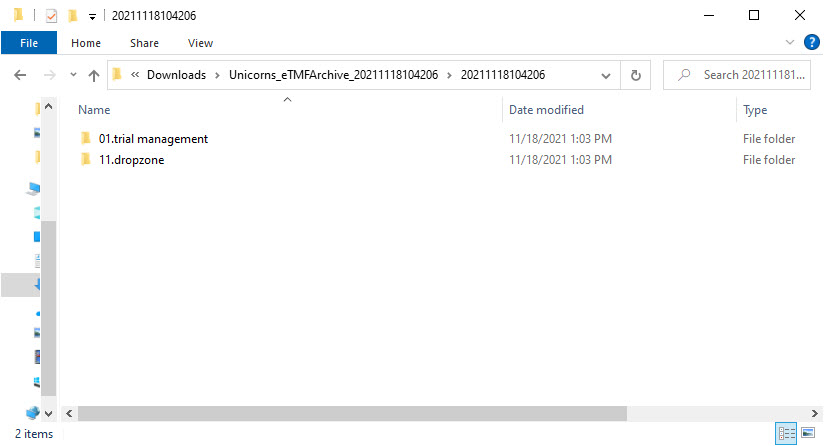
- Level 4 – this level includes:
- A folder with
ZoneID.SectionID.SectionNamefor each included section from the structure
- A folder with
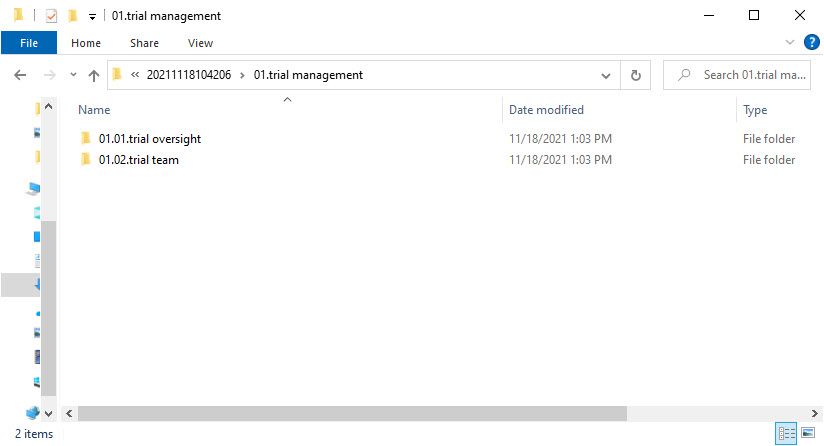
- Level 5 – this level includes:
- A folder with
ZoneID.SectionID.ArtifactID.ArtifactNamefor each included artifact from the structure
- A folder with
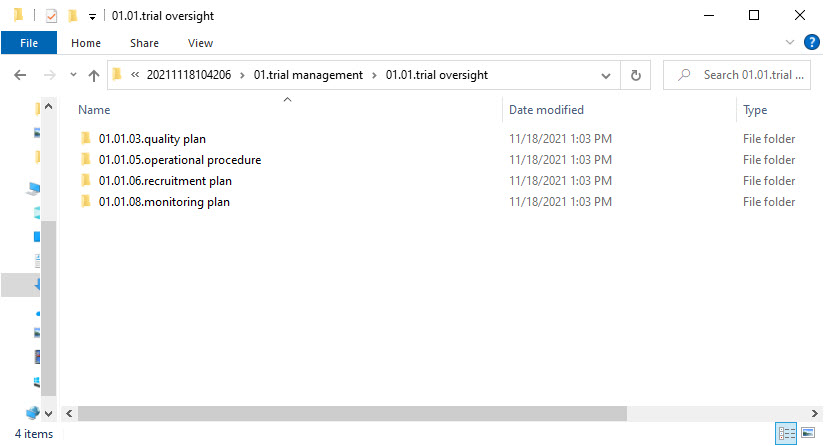
- Level 6 – this level includes:
- records filed to this artifact on trial level
- A subfolder for signed records*
- Subfolders for included countries with country code as their names
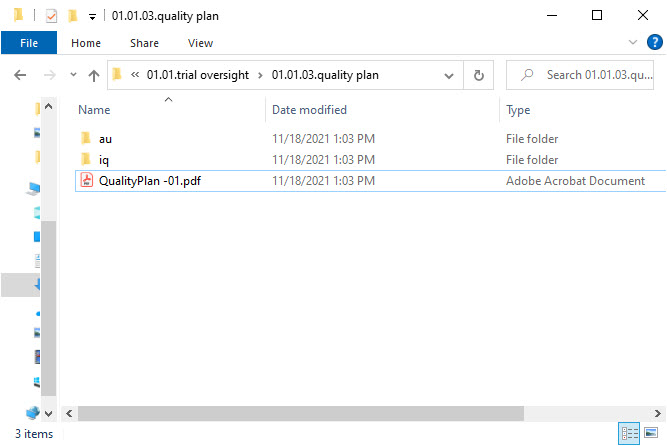
- Level 7 – this level includes:
- records filed to the artifact and linked to this specific country
- A subfolder for signed records*
- Subfolders for sites in this country with site names as their names
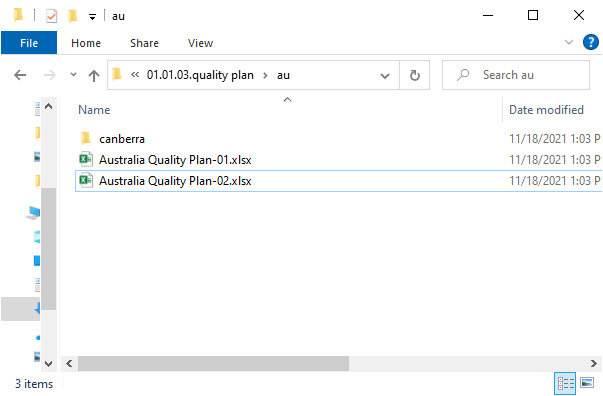
- Level 8 – this level includes:
- A subfolder for signed records*
- Records filed to the artifact and linked to this specific site
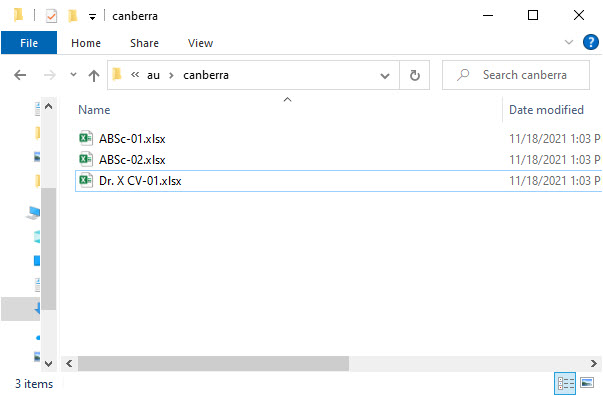
*The files that are signed by Viedoc Me users are only included when archiving the Investigator side of the TMF.
The zipped folder includes all the versions of records included in the structure. The name of the files will be as follows:
CurrentDocumentName-SystemVersion.extension, where:
CurrentDocumentNameis the latest record nameSystemVersionis the integer value of the version set by the system for this file
If there are multiple records with the same name filed to the same artifact and linked to the same levels, the system will add (n) as a suffix to the record name to ensure that all files are included in the zipped folder and no files are overwritten.
