Remote monitoring in Viedoc: a practical guide
Remote monitoring in Viedoc and how to optimize your study for it
Monitoring ensures that a trial is conducted in compliance with international regulations, standards and guidelines and is critical for the protection of the rights, safety and well‐being of trial participants and for the collection and analysis of data. Regardless of the location of the participating sites the need for and importance of monitoring is the same. There are a number of ways in which a clinical trial can be monitored including on‐site or off-site/remote procedures. This article focuses on the processes and procedures involved when conducting remote monitoring activities in Viedoc. Remote monitoring is an off-site review and evaluation of the study data, carried out by a team including monitors and medical reviewers at a remote location.
Remote monitoring in Viedoc
In addition to the source data verification which is typically conducted on site, the responsible monitor should review the study data on a regular basis. This activity is preferably performed remotely and is in Viedoc done by marking each reviewed form as “Clincial review”, indicating that the form has undergone review. The monitor can choose to review and mark each form individually or use the review console which allows for a more efficient handling of review. During the review process queries can be raised to prepare for the upcoming on-site visit.
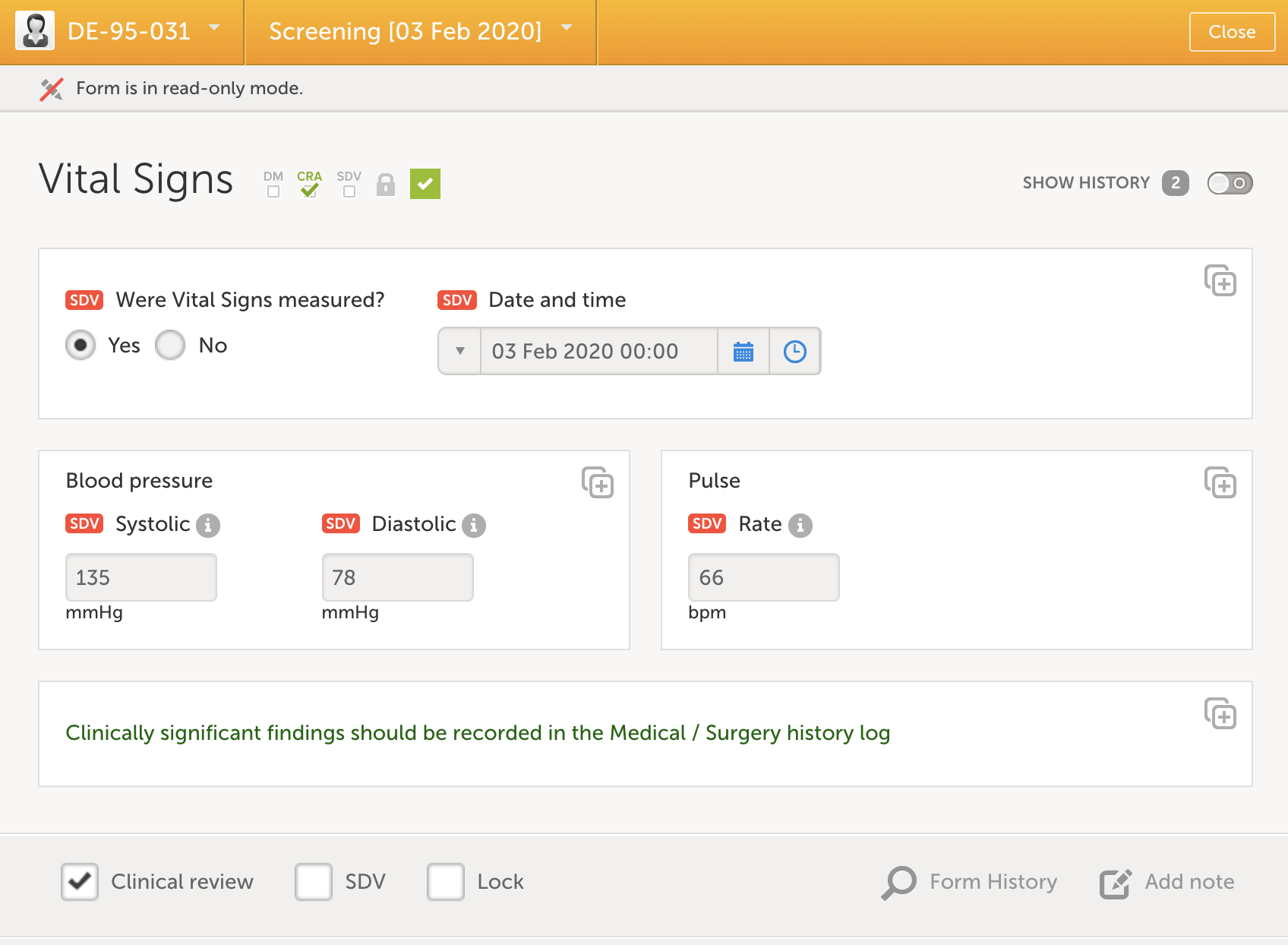
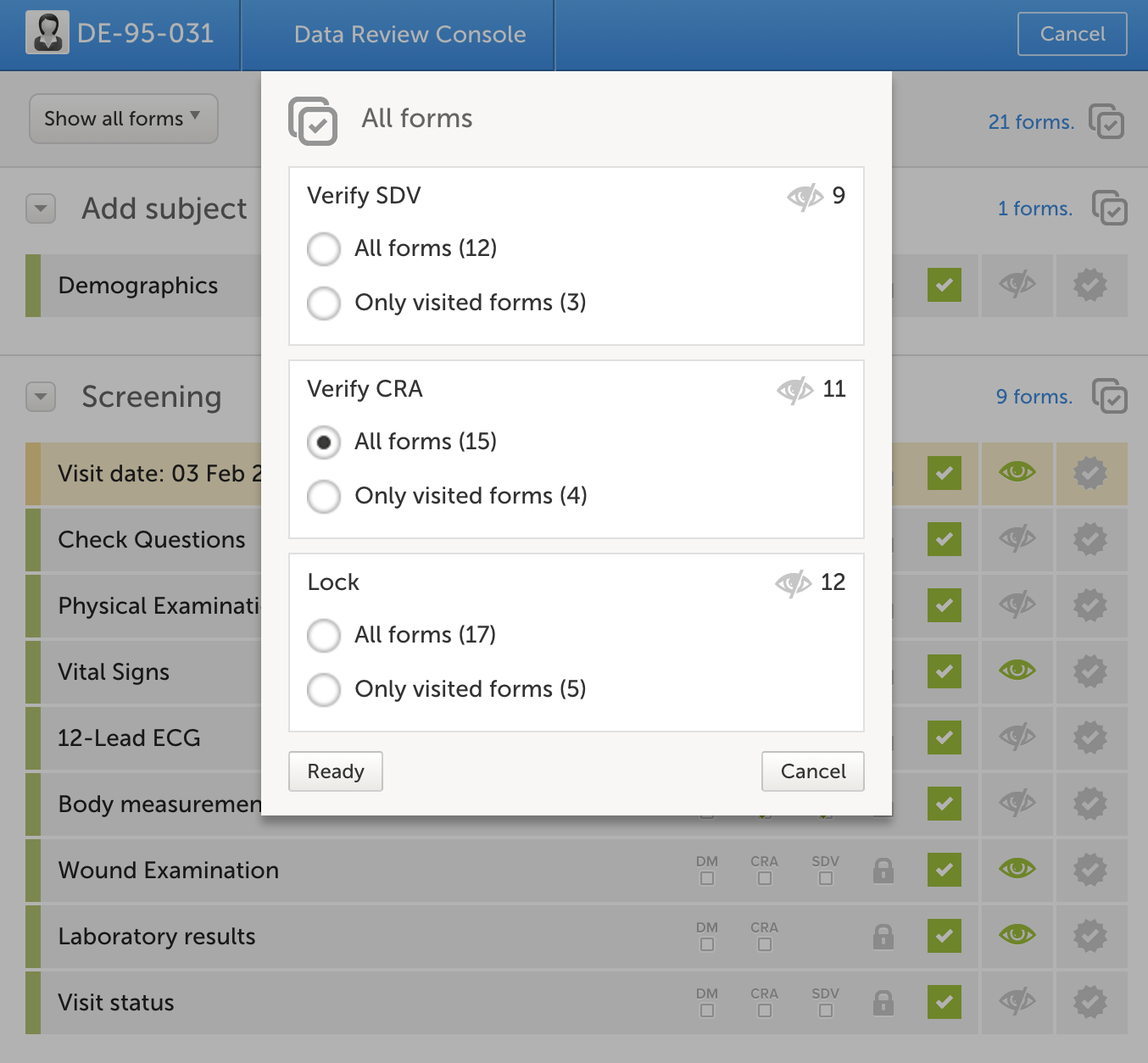
A more effective way of reviewing data
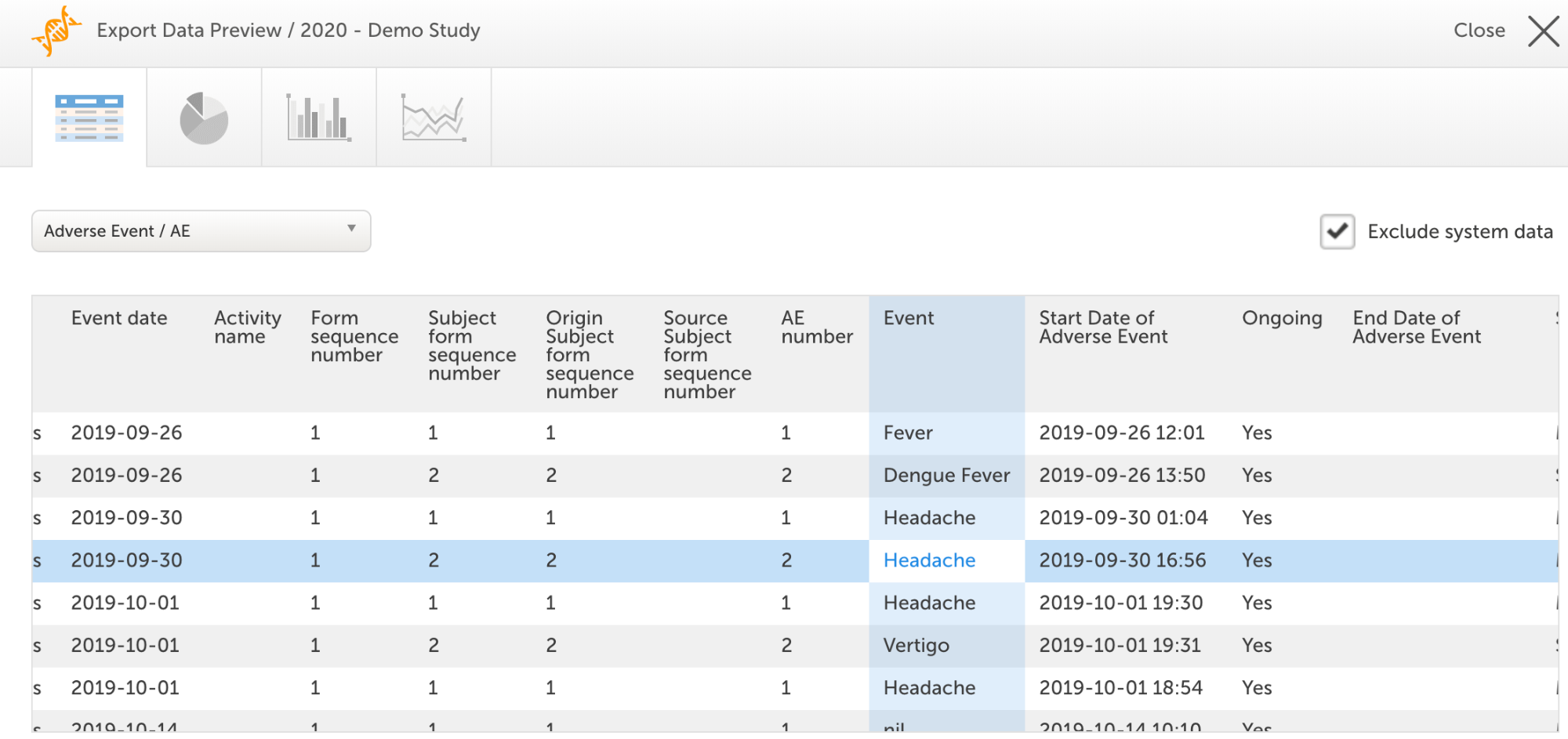
To facilitate the review even further the CRA can use the Data Preview page to display data from multiple patients at the same time. The selected data can be displayed in a table view or as simple graphs like pie or line charts or column bars.
How to optimize your study for remote monitoring
One way of increasing the amount of data that can be reviewed remotely is to allow for the site staff to upload source records directly into the eCRF, e.g. lab reports from local labs or other source records. Also, the more Viedoc “add-on” features you have for your study; i.e. randomization, allocation and logistics will allow you to review drug accountability ensuring the site has sufficient stock, and dosing and enrolment data. As data collection, randomization, drug dispense etc are all activities done in real time in Viedoc the monitor can be certain that what is reviewed is the accurate data.
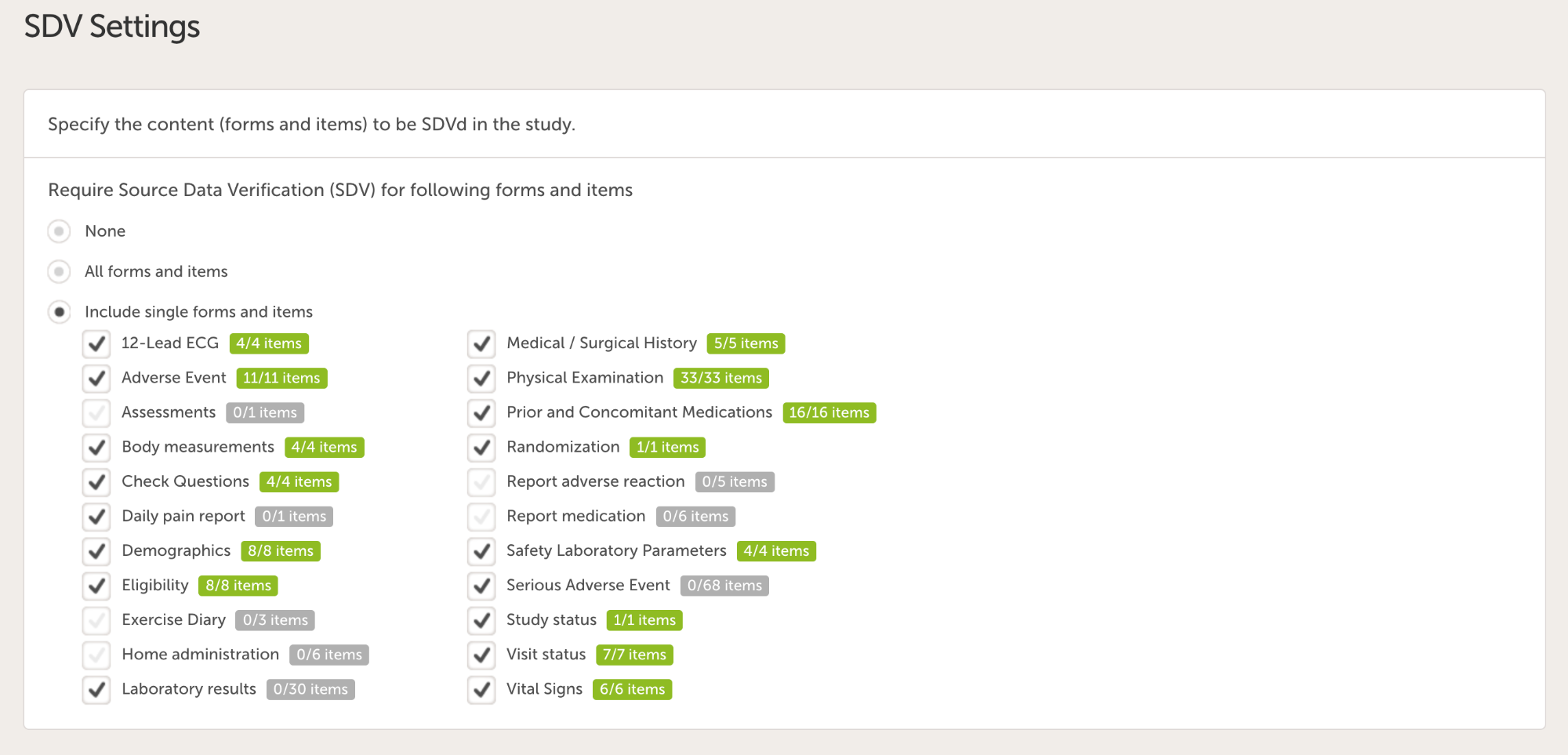
Targeted SDV
To further optimize what to focus on there is a possibility to define what items that need to be source data verified. This is done in Viedoc Designer and the following options are supported:
- No SDV
- All forms and items
- Individual forms and items (see image below)
Conclusion
The value of effective monitoring for clinical trials should not be underestimated. Viedoc remote monitoring capabilities can assist in ensuring reliable and accurate scientific results while adhering to local and international guidelines and maintaining patient safety throughout.
Enjoy your trial!
