Design ODM file structure
This lesson provides information about the Clinical Data Interchange Standards Consortium (CDISC) Operational Data Model (ODM) file structure and terminology, to help in interpreting the Viedoc study design ODM XML file structure and the data export output ODM XML file structure. Understanding this structure is useful when using the design version compare option to track changes between study design versions.
The ODM is a vendor-neutral, platform-independent format that facilitates data exchange and archiving. It includes metadata, clinical data, administrative data, and audit information, that is essential for study setup and execution.
Design ODM file data structure
In Viedoc Designer, the export function is used to export a study design to a Clinical Data Interchange Standards Consortium (CDISC) Operational Data Model (ODM) file. The attributes of each item in the ODM file, include data type, name, and Object Identifier (OID) which follow the ODM standard. The study design can be exported with or without CDISC SDM and Viedoc extensions. The CDISC SDM extensions follow the CDISC Study/Trial Design Model in XML (SDM-XML). SDM is an extension of ODM, and defines three key sub-modules - structure, workflow, and timing - permitting various levels of detail in any representation of a clinical study’s design.
Viedoc extensions: Vendor extensions are unique attributes in Viedoc that are not part of the ODM standard.
These attributes are highlighted with the prefix v4: in the exported ODM file:
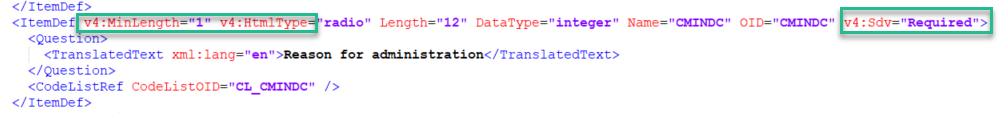
Note! When exporting designs from Viedoc Designer, you can exclude vendor extensions if you want to work with systems other than Viedoc.
The CDISC ODM file can be used for import into another project or another instance of Viedoc, for example a training instance. As the file is CDISC compliant, it can also be used in other systems equally compliant with CDISC standards.
Differences between a Design ODM file and a Clinical Data ODM file
The structure of exported clinical data has a hierarchy from high-level data down to individual items. It is important to understand the clinical data structure for exporting data, as it is structured in a way that is necessary for correct data handling. Each subject has multiple events, each event contains forms, item groups, and items.
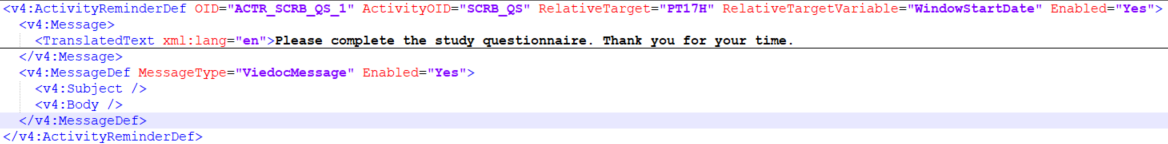
The diagram below shows the differences in structure between the Viedoc Design ODM file structure and the data export ODM file structure from Viedoc Clinic.
Note! When exporting a data ODM from Viedoc Clinic, the MetaDataVersion includes the data for ALL designs that have been used to collect data for any data point in that export.
General elements
GlobalVariables
Global variables include general summary information about the study.
StudyName: Defines the study name.StudyDescription: Brief description of the type of study.ProtocolName: Specifies the study protocol name.
BasicDefinitions
- Measurement unit: The physical unit of measure for a data item or value. The meaning of a MeasurementUnit is determined by its Name attribute. Examples include kilograms, centimeters, cells/milliliter.
MetaDataVersion
A metadata version defines the protocol, the types of study events, forms, item groups, and items that form the study data.
Examples:
Protocol
The protocol section is part of the CDISC SDM extension, and contains the study workflow information, for example, study entry and exit criteria, and trigger conditions for e-mails and timing, as well as study event references, which are the types of study events that are allowed to occur within the study.
In Viedoc, events can be split into multiple activities. This section also has all of the activity definitions and all of the form references to the forms in each activity:

StudyEventDef
A study event definition, StudyEventDef packages a set of forms. Within each study event definition, there are references to activities and forms, and within activities, there are references to forms. More information about how the form is set up is detailed in the form definition.
To find the information in a form definition: FormDef from a form reference: FormRef section, simply search for the relevant form definition, in this example, the DM form is the form referenced:
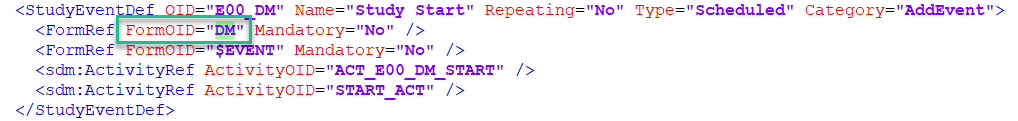

Select and expand the relevant form definition in the search results to view the form definition:
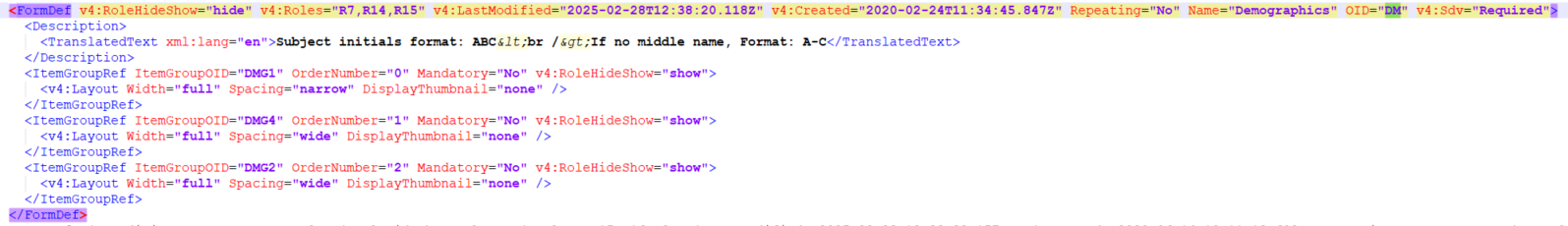
FormDef
A form definition, FormDef describes a type of form that can occur in a study.

The form definition will contain information about the item groups in the form as an ItemGroupRef. Similarly, if you want to view the Item Group definition information, you can perform a search as described above.
ItemGroupDef
An item group definition, ItemGroupDef describes a type of item group that can occur within a study, with the references to the individual items.These individual references are called ItemRef.
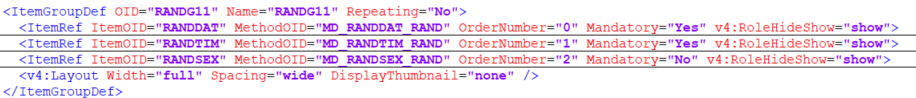
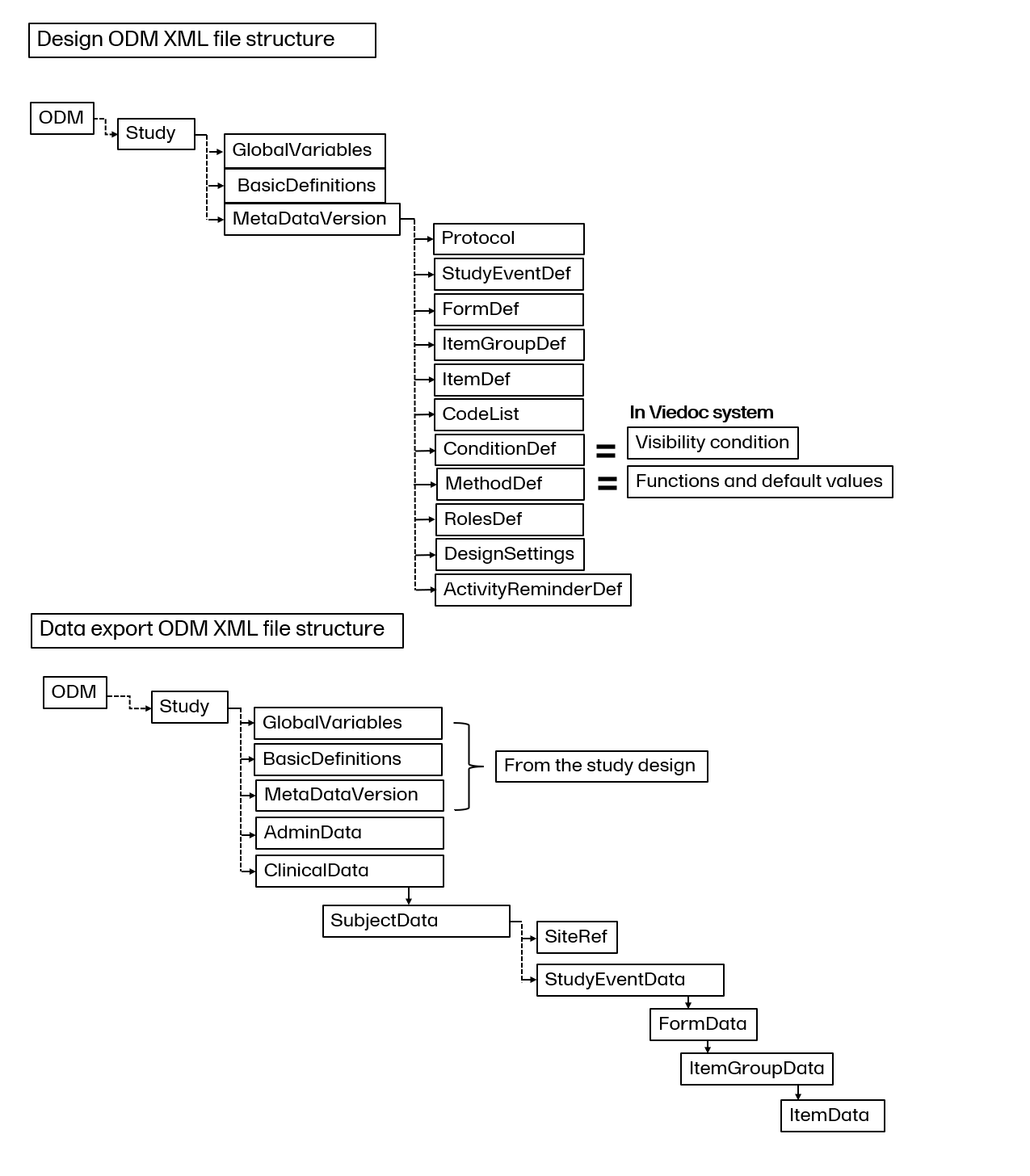
ItemDef
An item definition, ItemDef describes each item included in the study design. The item definition will also contain range checks, which correspond to any validation check added to the item. If a CodeListRef is contained within an item definition, it has a code list ID and is a reference to a CodeList:
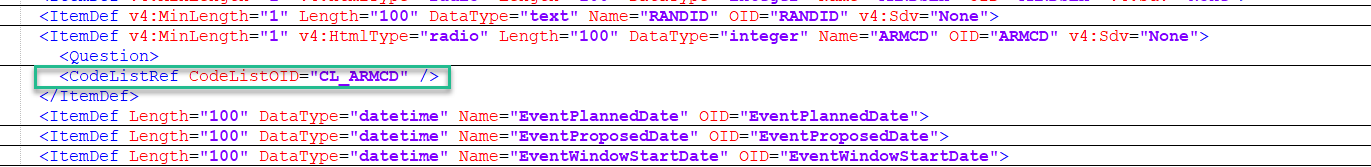
To locate the code list information, search for the code list OID in the code list reference.
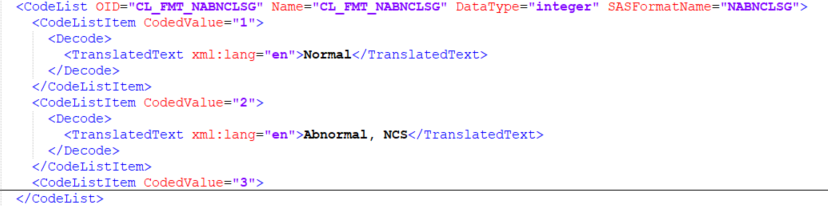
CodeList
A code list, CodeList defines a discrete set of permitted values for an item.
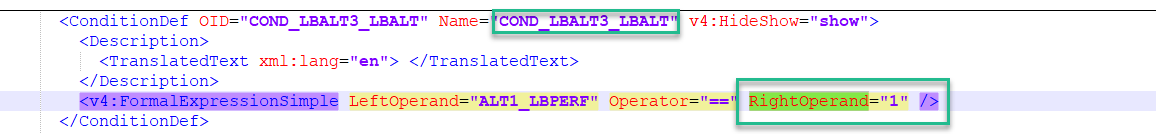
ConditionDef
A condition definition, ConditionDef defines a boolean condition. In Viedoc, the visibility conditions in the study design are converted to a condition definition.
In the simple visibility condition example below, if the item value =1, then the highlighted item will be visible:
There are also advanced visibility conditions which in the ODM file translates to FormalExpression followed by JavaScript code:
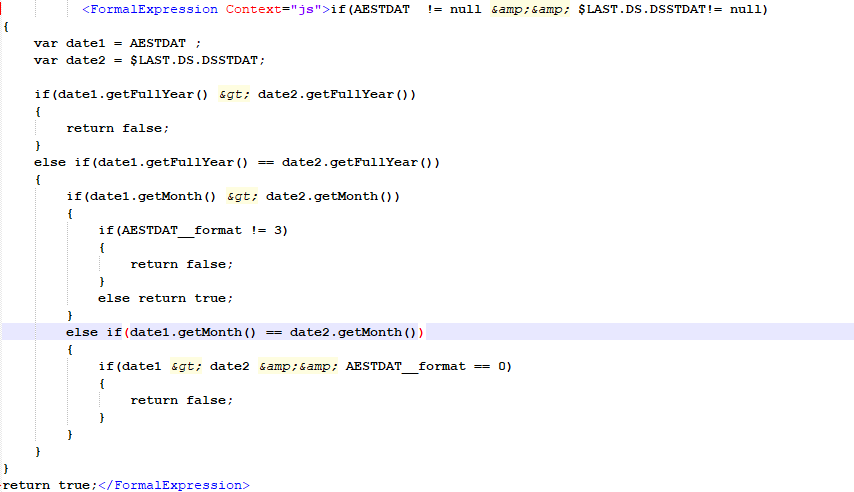
MethodDef
In the ODM file, method definitions, MethodDef are used for functions and default values in the design, and are important for interpreting the design. For more information, For more details about functions and how to use JavaScript in Viedoc, see default value and Viedoc provided functions in Using JavaScript in Viedoc.

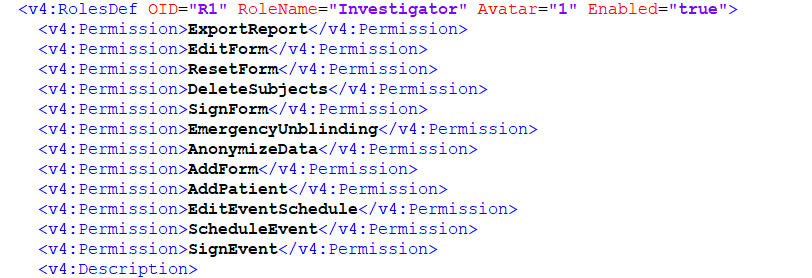
RolesDef
Roles definitions, RolesDef is a unique Viedoc vendor extension showing the different roles and associated permissions for the study.
DesignSettings
Design settings, DesignSettings is a unique Viedoc vendor extension showing selected study settings, if Source Data Verification (SDV) is selected for forms and items specified in the study design. This section will also have validation warnings and errors from the design.

ActivityReminderDef
The activity reminder definition is a unique Viedoc vendor extension showing the type of activity reminder and message for subject initiated events (Viedoc Me).