Clinical review, SDV, and Lock
Note that much of the information in this lesson is the same as in the lesson Data review and Lock.
Introduction
The requirements on data review and Source Data Verification (SDV) vary between studies. This lesson describes what is possible in Viedoc.
In this example, clinical review and SDV are tasks to be completed. The number of tasks to be completed is displayed in the orange task icon:
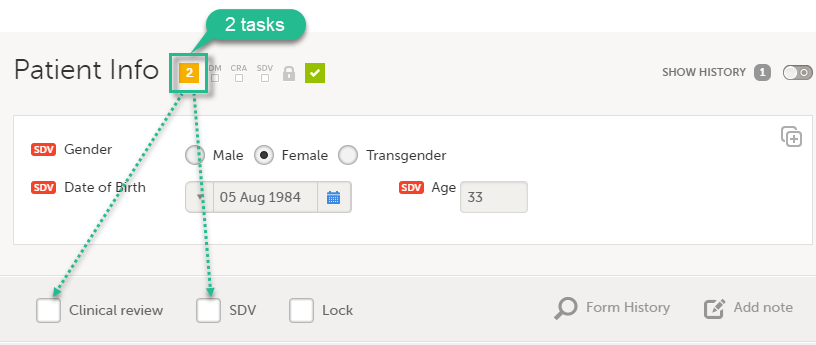
The orange task icon disappears when the checkboxes for clinical review and SDV are selected and the tasks are completed.
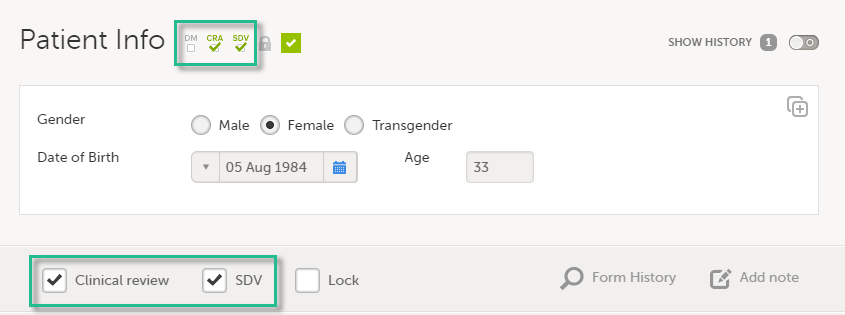
Note! If a user with edit permission is editing the subject card, you can still perform the clinical review and the SDV. And vice versa, the clinical review and SDV will not lock the subject for users who need to edit it.
Clinical review
The purpose of clinical review is to give the Monitor the possibility to mark forms as reviewed.
Marking a form as clinical reviewed can be performed in one of the following ways:
- At the bottom of each form, by checking the Clinical review checkbox.
- Batch-wise through the review console. Read more about the review console below.
Marking a form as reviewed does not mean that you are on-site having access to source data. It means that you have done a clinical review off-site of the content in the forms, and that you are prepared for your upcoming monitoring visit.
Note! If a form is edited after you have marked it as clinical reviewed, the review status breaks and the form must be reviewed again. The review task appears again in the orange task box icon.
SDV
Source data verification is normally the most time-consuming activity for the Clinical Research Associate (CRA), as it requires access to source notes. All forms that require SDV are highlighted with task(s).
SDV can be performed in one of the following ways:
- On item level, by clicking on the SDV flag for the item in the form.
- On form level, by selecting SDV at the bottom of the form.
Note! If there are items in the form that require SDV and are hidden due to data visibility conditions in the study design, you will not be able to apply SDV on form level. - Batch-wise through the review console. Read more about the review console below.
Note! If a form is edited after you have marked it as SDV, the SDV status is reset, and the form has to be SDV'ed again. The SDV task appears again in the orange task box icon. However, only the fields that were changed on the form are required to be SDV'ed again. These fields are clearly indicated with the red SDV icon.
SDV on item level
If the study has the setting for item-level SDV enabled, SDV can be performed for individual items in a form.
If the study design specifies that an item requires SDV, there will be an SDV icon next to the item in the form. The red icon indicates that SDV has not been performed. To perform SDV, simply click on the red icon. The icon then turns into a green SDV icon.
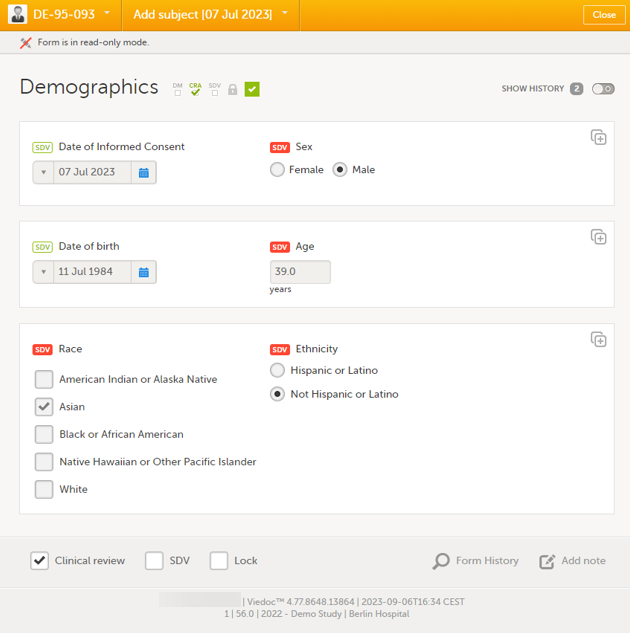
When all visible items that require SDV have been SDV'ed, the SDV checkbox at the bottom of the form will be automatically selected. And vice versa, if you select the SDV checkbox at the bottom of the form, all visible items that require SDV will be indicated with a green SDV icon.
Notes!
- If the form contains items that require SDV but are not visible to you, you will not be able to change the SDV status for the entire form.
- If an item is edited after the SDV has been performed, the SDV status is reset, and the item has to be SDV'ed again.
- When a study event form is SDV'ed, the event date form is automatically SDV'ed.
Lock
Locking a form
Locking data in a form can be performed in one of the following ways:
- At the bottom of every form, by selecting Lock.
- Batch-wise using the review console. Read more about the review console below.
Locking a form should only be performed if there are no more expected changes to that form, that is, if the data is clean.
| Important! Updates to the electronic Case Report Form (eCRF) are not applied to locked forms. If you are aware of any upcoming changes to the eCRF that potentially affect already saved and locked forms, make sure that these are unlocked before the new design version is published to the site. |
If all forms in all events for a subject have been locked, the subject card on the Selection page will be displayed with a lock icon, indicating all data is locked:
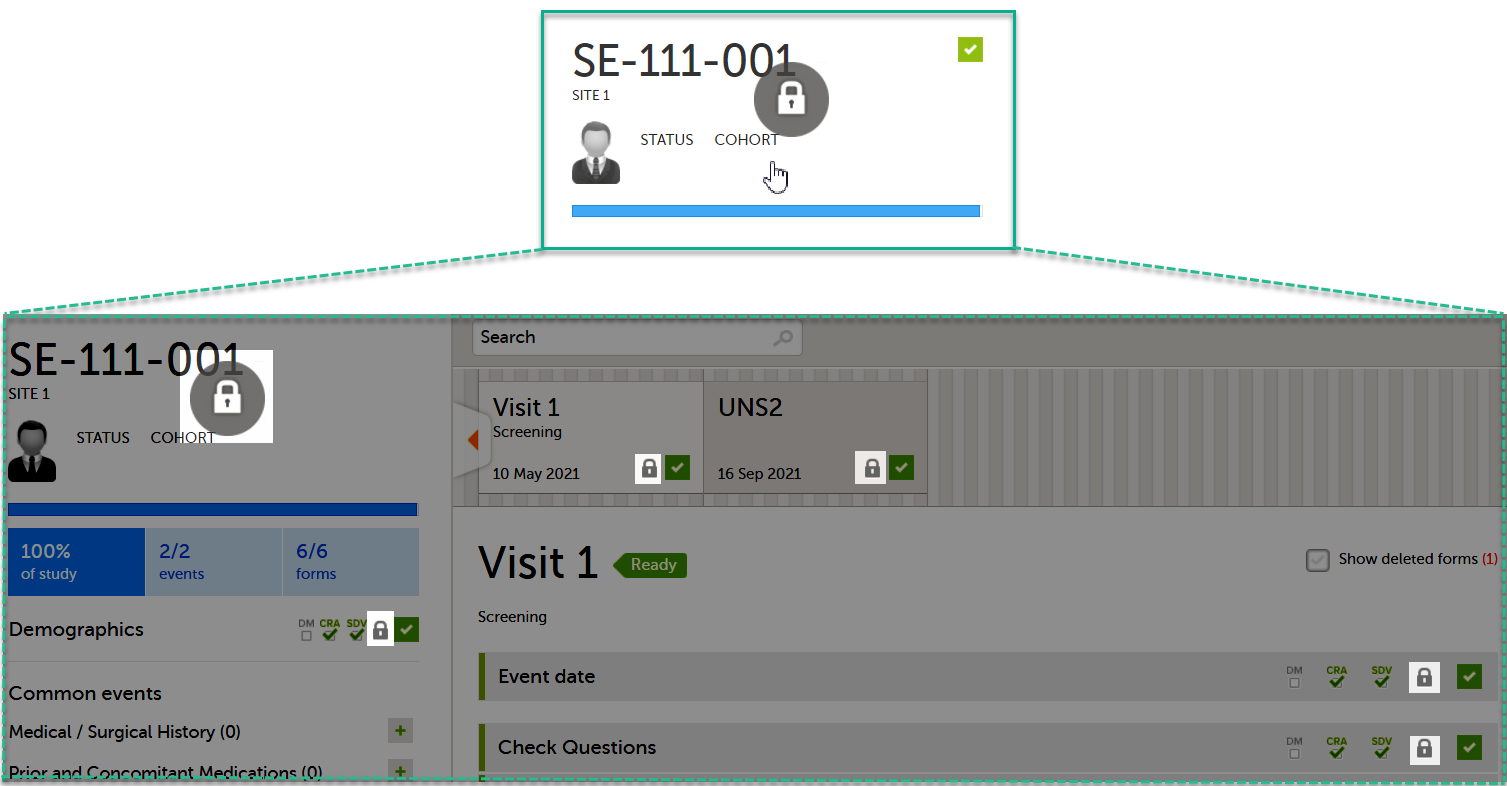
Unlocking a form
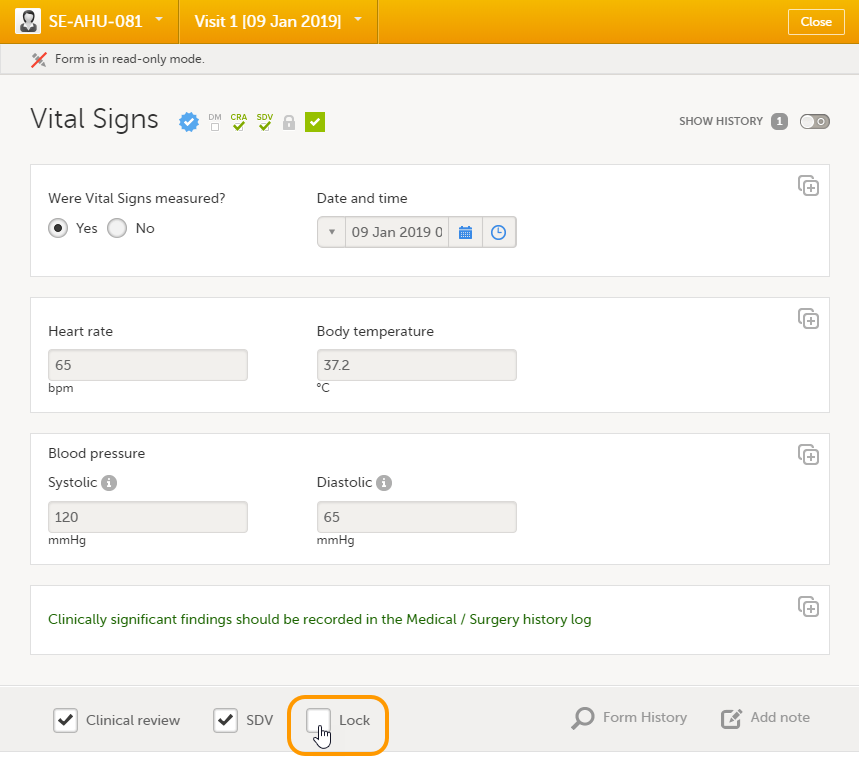
Regular clinic forms can be unlocked by clearing the Lock checkbox at the bottom of a form. Unlocking a form opens it up for editing by users with edit data permission (for example the Investigator).
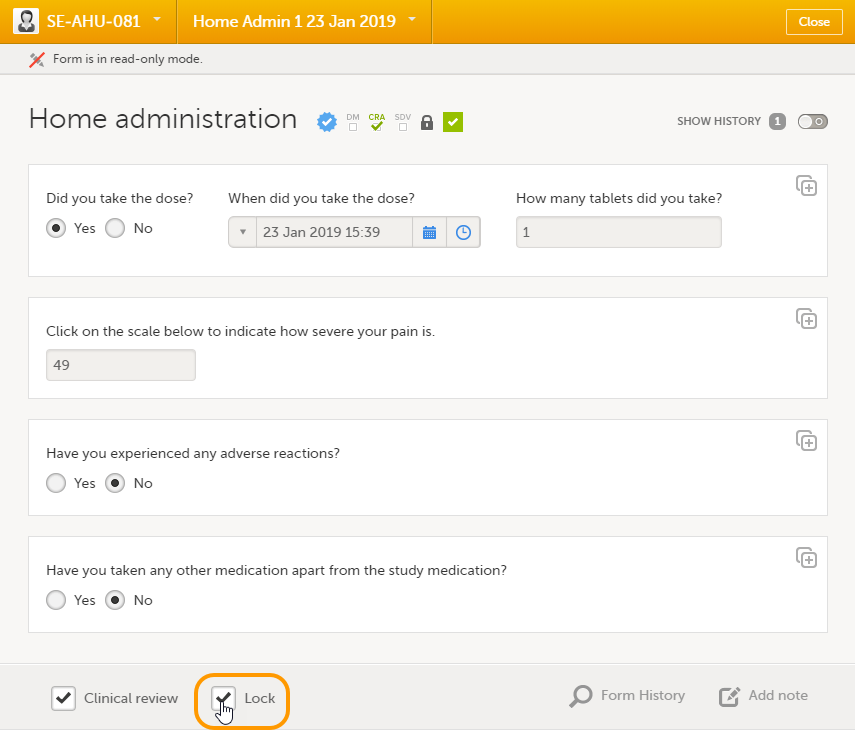
Locking/unlocking a subject-submitted (Viedoc Me) form
Subject-submitted (Viedoc Me) forms that are filled in by the subject are locked by default.
You may have the possibility to unlock a subject-submitted form if this option is activated for your study. In this case, the Lock checkbox appears at the bottom of the subject-submitted form. The form can be locked or unlocked by selecting or clearing the checkbox respectively. Unlocking a form opens it up for editing by users with edit data permission (for example the Investigator).
Data review console
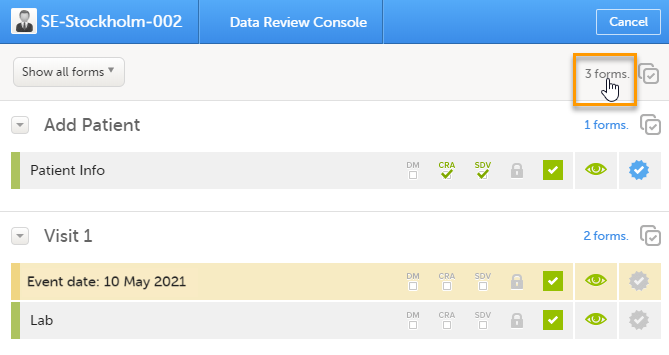
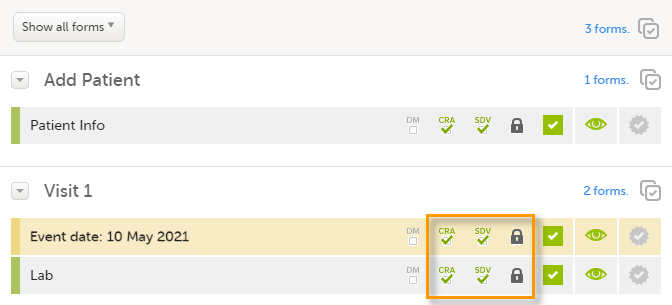
You can perform clinical review, SDV, and/or lock of the forms batch-wise, by using the data review console. To open the data review console, click the icon in the top right corner of the Details page.
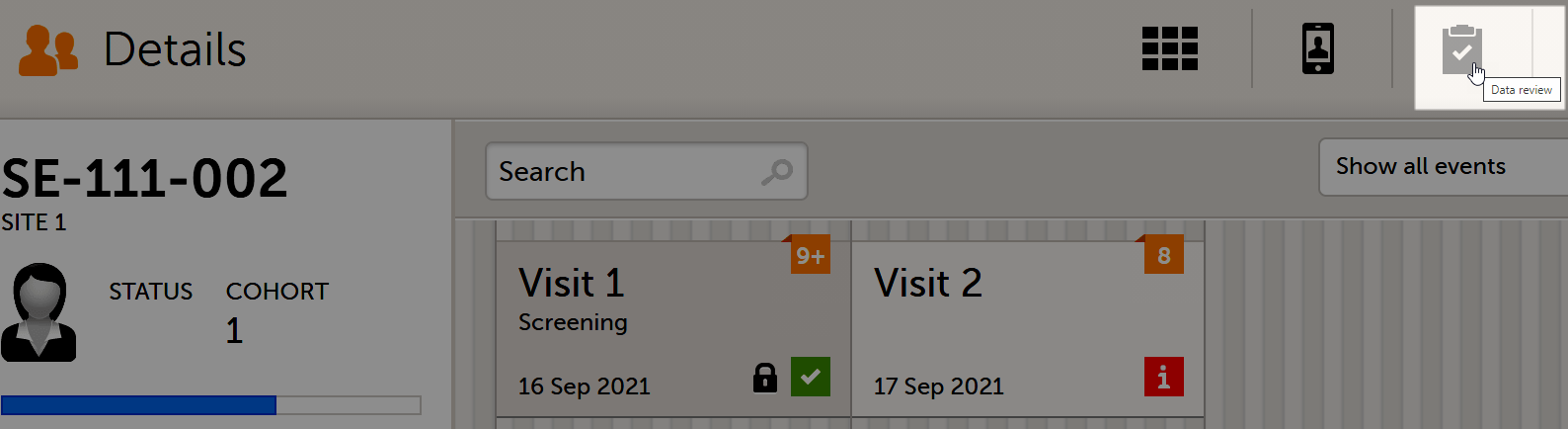
The data review console displays an overview of all forms of a subject that require data review, SDV, or lock. It shows which forms have been reviewed, SDV'ed, or locked. The green and grey eye icons help you identifying forms that you have not previously visited: the green eye icon marks the forms that you have already visited, the grey eye icon marks the forms that you have not visited yet.
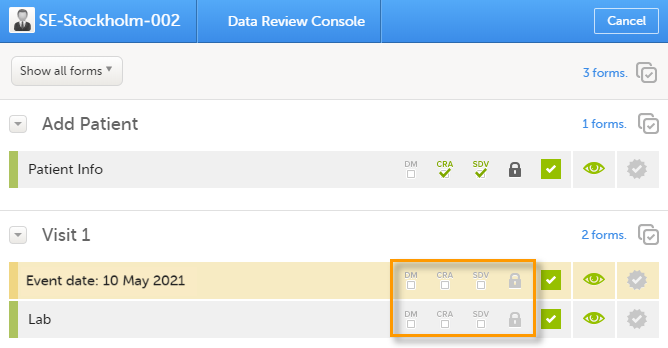
To review and/or lock the forms:
| 1 |
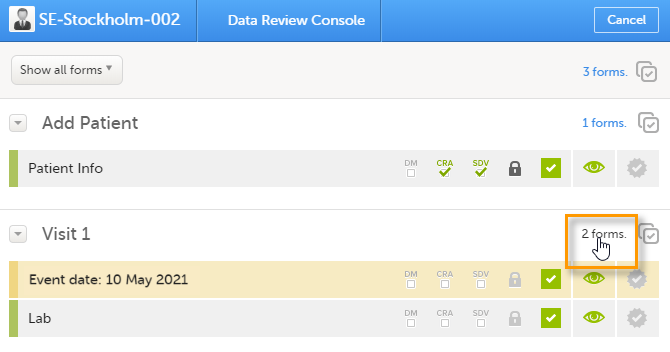
Select the form(s) to be reviewed in one of the following ways:
|
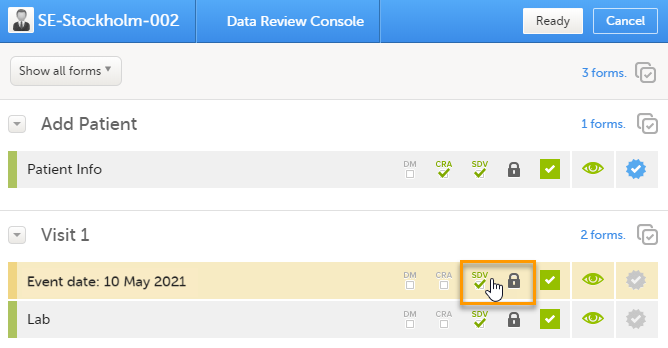
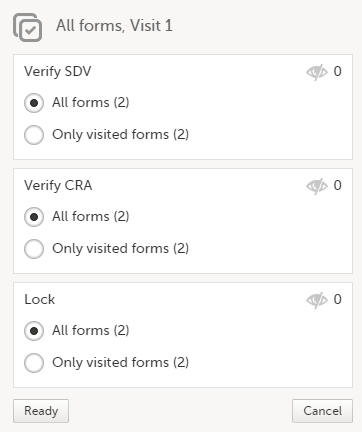
| 2 |
In the dialog that opens, select Clinical review, SDV, Lock as needed, and click Ready: The status of the selected forms is updated according to the selected actions. |
If any of the marked forms have not been visited by you before, you will be asked whether you want to continue with the action or not. If you choose to continue, the forms will be marked according to your selections, that is, the system will not prevent you from marking unvisited forms as reviewed, SDV'ed, or locked.
Note! If the study has the setting for item-level SDV enabled, and a form contains items that require SDV but are not visible to you, you will not be able to change the SDV status for the entire form.
Study status and metrics
The current workload can be checked on the Study status or the Metrics pages.