Viedoc Study Build Service – RTSM Considerations
The purpose of this document is to give a general high-level description of important things to consider when a customer is planning to use Randomization and Trial Supply Management (RTSM) in a study and use Viedoc’s study build service.
General
When Viedoc Technologies is responsible for the study build, the build team will configure the randomization and kit allocation settings in Viedoc Designer according to the protocol and instructions from the customer. Input factors/stratification variables and randomization outcomes (both blinded and open) are specified to achieve the requested randomization and kit allocation workflow. The customer will be provided with a template for the randomization list (and kit list if Viedoc Logistics is used). A user, normally the customer statistician, will be given access as an “unblinded statistician” and be responsible for uploading the randomization list to Viedoc. Viedoc will provide the instructions and guidance as necessary for the user to be able to upload the list.
Randomization
Randomization Settings
The study build team at Viedoc Technologies will configure the randomization according to the protocol and add the input factors and outcomes needed and in agreement with the customer. The randomization/allocation list can be uploaded per site, per country or per study. If nothing else is specifically communicated or mentioned in the protocol, the setting used will be one randomization/allocation list per study. Viedoc has support for both static and dynamic randomization.
Randomization List
If a static randomization is used, the customer is provided with a template randomization list by the Viedoc representative. The randomization list will then be generated and uploaded by the customer, normally the randomizing statistician. This user would be invited with the role “unblinded statistician” to upload the list. The list must be in .xlsx format and must include the same column headers and code list values as communicated in the randomization list template. If the requirements are not met, the upload will fail.
Updated randomization lists, for example, lists with changed treatment ratio or additional randomization slots can be uploaded during the study by the same user.
It is strongly recommended that the customer creates both a dummy list and a production list. If no dummy list has been provided to Viedoc at the time of the first CRF review, Viedoc can add its own dummy list for the purpose of testing the randomization functionality and unblinding.
Unblinding
If the study is blinded, emergency unblinding permission can be added in the EDC. By default, this permission is enabled for the investigator role, but this can be customized per the customer’s request.
Viedoc Logistics
Display of Kit Information in Viedoc
Items associated with each kit can be populated in the Viedoc Logistics interface, the kit list export, and/or in the allocation form in the EDC. All items to be populated must be included in the kit list uploaded to Viedoc.
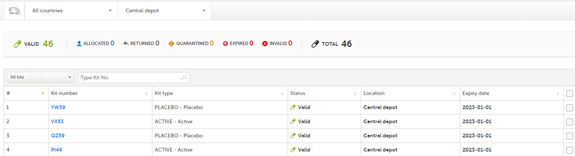
Included in the Viedoc Logistics Interface
The kit number and kit type are mandatory to display, whereas expiry date is optional (but recommended).
The following image shows the view for a Study Supply Manager:
Included in the Allocation Form in the EDC
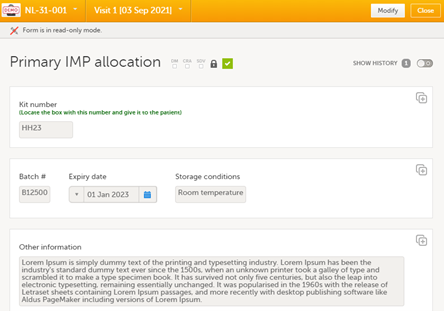
In addition to the kit number, an option is to display additional supportive items in the allocation form in the EDC when kits are allocated. This would then be available for the site users allocating the kits and anyone with read access to the form.
The following image shows a saved allocation form in the EDC with kit details already populated:
Included in the Kit List Export
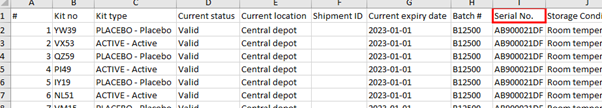
Supportive items can also be included in the kit list, being available when exporting kit data from Viedoc Logistics. These items do not necessarily need to be included in the kit allocation form in the EDC.
The following image shows the inclusion of the serial number in the kit export from Viedoc Logistics.
Kit Allocation Settings
There are options on how to configure the kit allocation. For increased flexibility the kit allocation setting can allow to enable “replace allocation” and/or “undo allocation”:
- “Replace allocation” is used when the subject would need a new kit when the initial kit can for some reason no longer be used. The initial kit would still have the status “allocated”.
- “Undo allocation” can be used for undoing an allocation. The initial kit is then made available for new allocations and the status of the kit is returned to “valid”. This is only recommended for open studies.
By default, the build team will enable “replace allocation” and disable “undo allocation”.
The CRF can also be set up to allocate multiple kits per visit when required.
Kit List
Just like for the randomization list, the kit list is uploaded by the customer. The user with the role Unblinded Statistician will be able to upload the list. The list must be in .xlsx format and must include the same column headers and code list values as communicated in the kit list template. If the requirements are not met, the upload fails.
Once a kit list has been uploaded, all kits in the list will be placed in the central depot in Viedoc Logistics and can thereafter be distributed to sites as applicable.
For kit numbers, it is recommended to use non-sequential series (for example random numbers or a combination of letters and numbers) to make it as difficult as possible to figure out the treatment in a kit (for example in a scenario where a subject has been unblinded and the content of several kits have become known).
Updated kit lists, with additional kits, can be uploaded during the study. However, the format of the kit list must be the same throughout the trial.
It is strongly recommended that the customer creates both a dummy list and a production list. If no dummy list has been provided to Viedoc at the time of the first CRF review, Viedoc can add its own dummy list for the purpose of testing the allocation functionality.
User Access
Study Supply Manager
When using Viedoc Logistics, one or several users will serve as Study Supply Manager. This is a role that will have access to blinded information, that is, they will see the contents of each kit. The Study Supply Manager will also be able to set thresholds for low stock alerts on a study level and site-specific alerts. They will also be able to receive emails for low stock alerts. The role Study Supply Manager is usually assigned to one or several users at a depot//manufacturer or to one or several unblinded users at the sponsor/CRO.
Site Supply Manager
Each site will have one or several users with access as Site Supply Manager. This user will be able to receive kits in Viedoc and also change status of the kits as appropriate. For blinded studies, this user will not see blinded information.
Other User Roles
Other roles, such as read-only access for the Monitor can be set up as requested.
More information on how to use Viedoc Logistics can be found here.
