Customizing a template
Introduction
The eTMF template file defines which zones, sections, and artifacts that your eTMF will have. Furthermore, it defines the permissions associated with the eTMF user roles.
The eTMF template is implemented in an Excel file.
As an eTMF Manager, you have the permission to import, select, instantiate, export, rename, and delete templates.
With your Viedoc eTMF license, a baseline template is provided. This template is not intended to be used as it is, but to be adapted to your organization's needs. See Viedoc-provided templates for more information.
To access the templates in Viedoc eTMF, click the Templates tab at the top of the page:
In Viedoc eTMF, there are two types of templates:
- Organization template - available for all studies within your organization
- Study template - available only for the specific study
It is recommended that you adapt the eTMF template to your specific documentation landscape. For example, you can customize, add, or delete zones, sections, and artifacts.
However, the following requirements must be fulfilled for Viedoc eTMF to successfully validate the template:
- The template must include nine sheets, in any order.
- The sheet names must not be changed.
- All columns in all sheets are mandatory, which means that they must be present. However, some columns can be left empty. For more information, see the following sub-sections.
- Some columns must include specific values. For more information, see the following sub-sections.
- All sheets must have the same number of rows, that is one row per artifact.
Note! If the following special characters (\/:*?<>|"\r\n) are used in the TMF template, they will be replaced by an underscore (_) during archive generation to ensure the archive completes successfully.
The V 3.2.1 sheet
This sheet is based on the TMF Reference Model. Zones, sections, and artifacts can be customized, added, and/or deleted.
These are the requirements for the columns:
| Zone # | Must be unique |
| Zone Name | Must be unique |
| Section # |
Must be unique It consists of two digits that represent the zone number that the section belongs to, followed by a separator and the section number. |
| Section Name | Must be unique within the zone |
| Artifact # |
Must be unique It consists of two digits that represent the zone number, followed by a period (.), then two digits for the section number, a period (.) and the artifact number. Example: |
| Artifact name | Must be unique within the section |
|
Alternate names (artifact also commonly known as) |
This is an alternative name for the artifact. This column is optional. If it exists, its value can be left empty. Note! This column is not currently mapped to system functionality. It is not currently possible to change it in maintenance mode. |
| Definition / Purpose |
A free-text description of the artifact To view the definition in Viedoc eTMF, go to the TMF structure tab, navigate to the artifact, and select View. 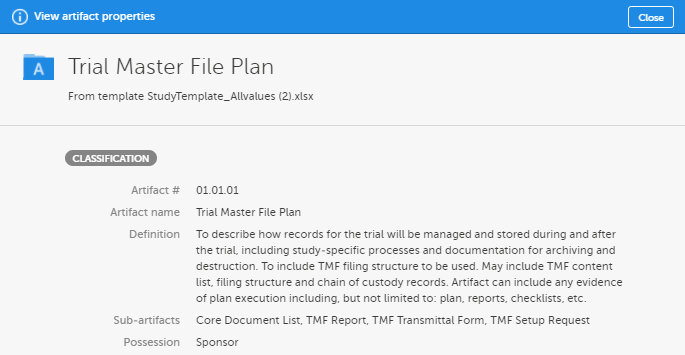
This column must have values. |
| Sub-artifacts |
A newline-separated list of sub-artifacts To view the list of sub-artifacts in Viedoc eTMF, see above. Sub-artifacts can be used by eTMF users to further classify documents. This column can be left empty. |
|
Core or Recommended for inclusion ICH Code |
These columns are related to GCP. These columns can be left empty. Note! This column is not currently mapped to system functionality. It is not currently possible to change it in maintenance mode. |
| Unique ID Number |
An optional unique ID number for the artifact. This number is validated as follows by Viedoc eTMF:
|
|
Sponsor Document Investigator Document |
These columns define what side of the TMF the artifact is: sponsor or investigator (according to GCP). The values can be These columns must have values. |
|
Process Based Metadata - Number Process Based Metadata - Name |
These columns define trial processes that artifacts can be linked to. This can be useful for trials where records are filed across multiple zones. Note! These columns are not currently mapped to system functionality. It is not currently possible to change them in maintenance mode. |
|
Trial Level Document Trial Level MILESTONE/EVENT Country/ Region Level Document Country Level MILESTONE/EVENT Site Level Document Site Level MILESTONE/EVENT |
These columns define which level (Trial/Country/Site) the document should be filed to and which milestones correspond to each. When a Trial/Country/Site level document in the V 3.2.1 sheet in the template is set to:
|
| Dating Convention |
Defines the dating convention that is used in the metadata of documents uploaded to Viedoc eTMF This column can be left empty. If it is empty, the default dating convention will be the version date. If you select New in the Dating convention field in the Edit artifact window, you can, for example, enter an expiration date as the dating convention. |
The Viedoc extensions sheet
This sheet contains Viedoc-specific properties for each artifact.
These are the requirements for the columns:
| Artifact # | Unique artifact number as defined on the V 3.2.1 sheet. |
| Sign |
This column is not yet used in Viedoc eTMF, but it must have values. The following values are accepted: |
| Applicable in Trial |
Defines if the artifact is applicable at trial level. The column must have values. The following values are accepted: |
| Applicable in Country |
Defines if the artifact is applicable at country level. The column must have values. The following values are accepted: |
| Applicable at Site |
Defines if the artifact is applicable at site level. The column must have values. The following values are accepted: |
| Metadata properties |
Additional metadata for the artifact. This column can be left empty. To view the additional metadata in Viedoc eTMF, go to the TMF structure tab, navigate to the artifact, and click View. 
|
| File formats |
A pipe-separated list of accepted file formats for the artifact. Examples:
|
| Accept blinded data |
This column is not yet used in Viedoc eTMF. It defines if blinded data is accepted for the artifact. The following values are accepted: |
| Accept privacy data |
This column is not yet used in Viedoc eTMF. It defines if privacy data is accepted for the artifact. The following values are accepted: |
The Viedoc milestones sheet
This is an optional sheet. It includes Viedoc-specific properties for each milestone.
If this sheet is not provided, the system will create a list of milestones, under the group Other, based on the following specified milestones in the V 3.2.1 sheet:
- Trial Level MILESTONE/EVENT
- Country Level MILESTONE/EVENT
- Site Level MILESTONE/EVENT
These are the requirements for the columns:
| Id | Unique milestone Id. This column is mandatory. |
| Name | Unique milestone name. This column is mandatory. |
| Group | One of the four milestone groups defined in the CDISC Reference Model. This column is mandatory, and the following values are accepted: Start UP, Study Conduct, Close Out, Other |
| Trial description | Description of trial-level documents this milestone includes. This column can be left empty. |
| Country description | Description of country-level documents this milestone includes. This column can be left empty. |
| Site description | Description of site-level documents this milestone includes. This column can be left empty. |
Role sheets
The role sheets define the permissions associated with each of the eTMF roles.
These are the role sheets:
- Role SPONSOR-STUDY
- Role SITESTAFF
- Role SPONSOR-COUNTRY
- Role SPONSOR-SITE
- Role SPONSOR-REVIEW
- Role SPONSOR-DM
- Role SPONSOR-UNBLINDED
These are the requirements for the columns:
| Artifact # | Unique artifact number as defined on the V 3.2.1 sheet. |
| Study |
Defines the permission of the role on study/trial level. The following values are accepted:
|
| Country |
Defines the permission of the role on country level. The following values are accepted:
|
| Site |
Defines the permission of the role on site level. The following values are accepted:
|
