Overview of Viedoc PMS Designer
Introduction to Viedoc PMS
Viedoc PMS is the product on the Viedoc platform that can be used for Japanese Post Marketing Surveillance (PMS) studies. It fulfills all requirements of a PMS study, supports collection of data in booklets, and supports the process of sharing data between site (clinic) and sponsor via the submit-receive-return Kaifu function.
PMS studies are built in Viedoc Designer. Viedoc Designer is where you perform the technical part of a study build, either from scratch or by importing a design from a previous project. A design consists of the study forms, the booklets workflow, study roles, and other configurations and settings, as described further in this curriculum. The following sections describe the specific steps essential to build and design a PMS study.
For more information on how to design a study, see Viedoc Designer User Guide.
Clinic side versus sponsor side
In Viedoc PMS, the database is shared by two different sides, a clinic side and a sponsor side. The database contains two versions of each data set, one version that is displayed to the clinic side users and one version that is displayed to the sponsor side users. On the clinic side, typically the Investigator enters subject data, while on the sponsor side the Data Manager typically reviews the data and archives (freezes) the data.
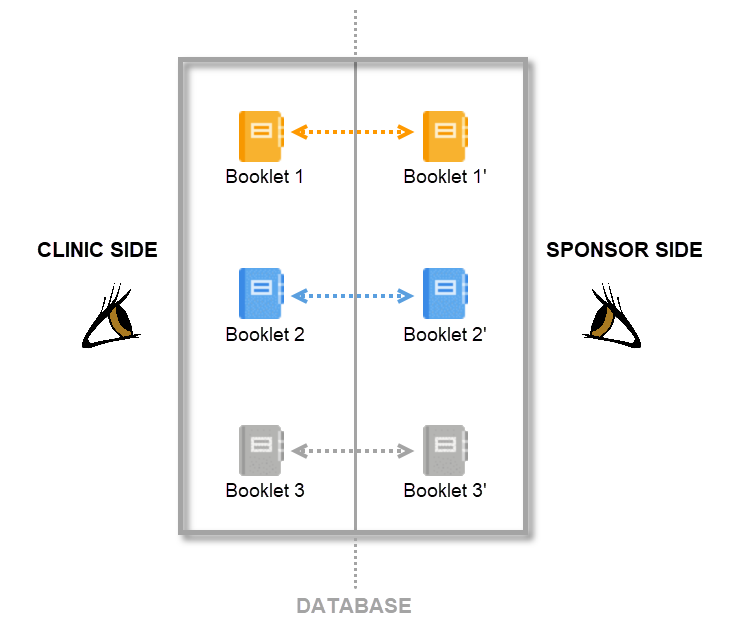
Booklets
By mirroring the data collection and review process via booklets, Viedoc matches the workflow of a Japanese PMS study. A booklet can be seen as a compilation of data being collected during a specific period of time rather than during a specific event date, which is more typical in clinical trials.

1.Subject details
2.Overview of booklets
3.Content of selected booklet
4.Details of selected booklet
The send/receive/return process for handling booklets (Kaifu)
Sending and receiving data on request is a fundamental requirement for a Japanese PMS study. Viedoc PMS offers support for sending and receiving booklets between site and sponsor, a process referred to as Kaifu. In the Kaifu process, the clinic user chooses when to share data with the sponsor and the sponsor side user chooses when to receive the data. It should be noted that the sponsor side user does not have access to any data entered in a booklet until the booklet has been shared by the clinic through the submit function, and a receive action has been actively performed by a user on the sponsor side.
For more information, see Overview of the submit-receive-return process
For more information about PMS operations for Clinic side and Sponsor side users, please see the following User Guides:
