A use case for working with reference data
This lesson provides a use case for working with reference data in Viedoc Designer, Viedoc Admin, and Viedoc Clinic.
Introduction
About reference data
Viedoc offers support for adding reference data that will be automatically populated to specific forms. When reference data are configured for your study, it is not necessary to fill in reference values for each subject in each form separately.
It is possible to configure different sets of reference data that will be populated to the form based on:
- factors that can affect the reference data, such as age or gender
- reference data source, such as a lab
- site
- date
Terminology
| Term | Definition |
|---|---|
| Reference data source |
An institute that provides reference data, for example a lab. |
| Reference data scope | A mapping of the items that should be automatically populated with reference values, and the factors that they should depend on. One or more reference data scopes can be configured in Viedoc Designer > Global Settings, as set(s) of variables and factors (see definitions below). |
| Factor | A parameter that affects the reference data, for example a subject’s sex. Factors may affect the normal range for a test result. |
| Variable | A specific measurement to be carried out. |
| Date factor | The date the sample was collected or the measurement carried out. This field determines on which date the reference data to be populated are based, for example when the event date is not the same as the collection or measurement date. |
| Target type | An item of a certain type of information that a reference data source can provide for a specific variable (such as range, unit, low/high values, etc.). Any number of target types can be defined by the user. |
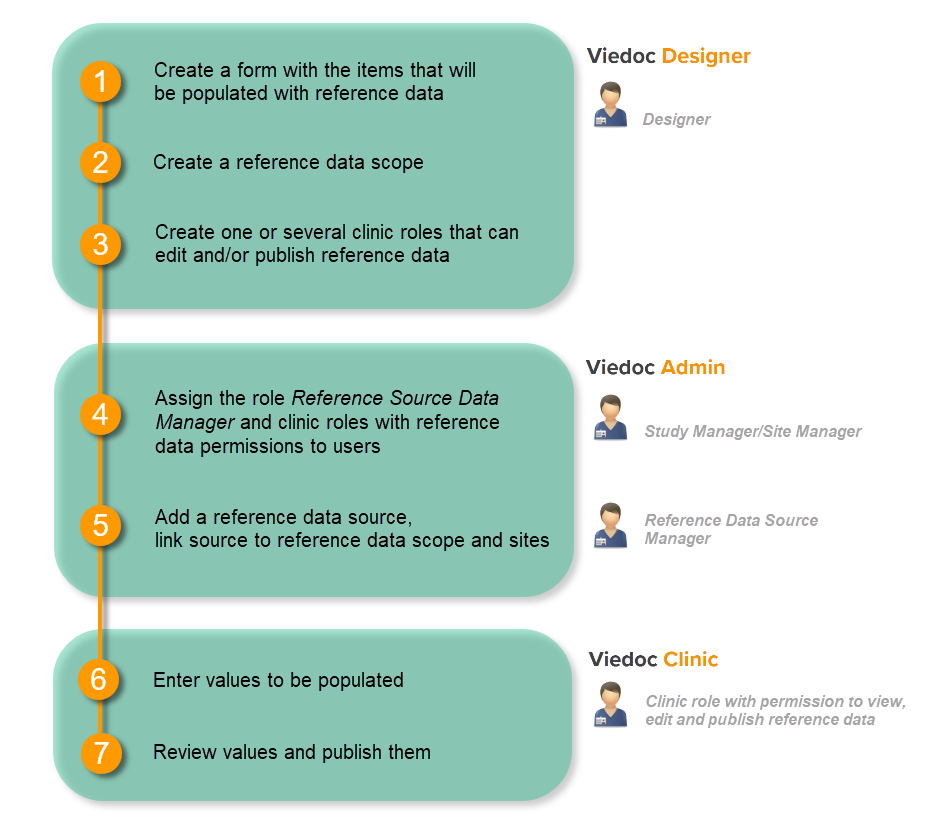
Workflow
Reference data are configured in Viedoc Designer, Viedoc Admin and Viedoc Clinic. The schematic below depicts what different steps need to be taken, and which roles have permission to perform these steps.
 http://help.viedoc.net/l/5cf069/en/
http://help.viedoc.net/l/5cf069/en/
This is a single-sourced file that should have the following content:
Introduction to reference data, description of the workflow.
For more detailed instructions regarding these steps, see:
- Configuring reference data scopes in Viedoc Designer
- Managing reference data sources in Viedoc Admin
- Working with reference data in Viedoc Clinic
For a video tutorial on how to work with reference data, see
Objective of this lesson
This lesson illustrates an example of configuring reference data in Viedoc Designer, Viedoc Admin, and Viedoc Clinic. It also shows how reference data are populated to the subject forms in Viedoc Clinic.
Working with reference data - an example
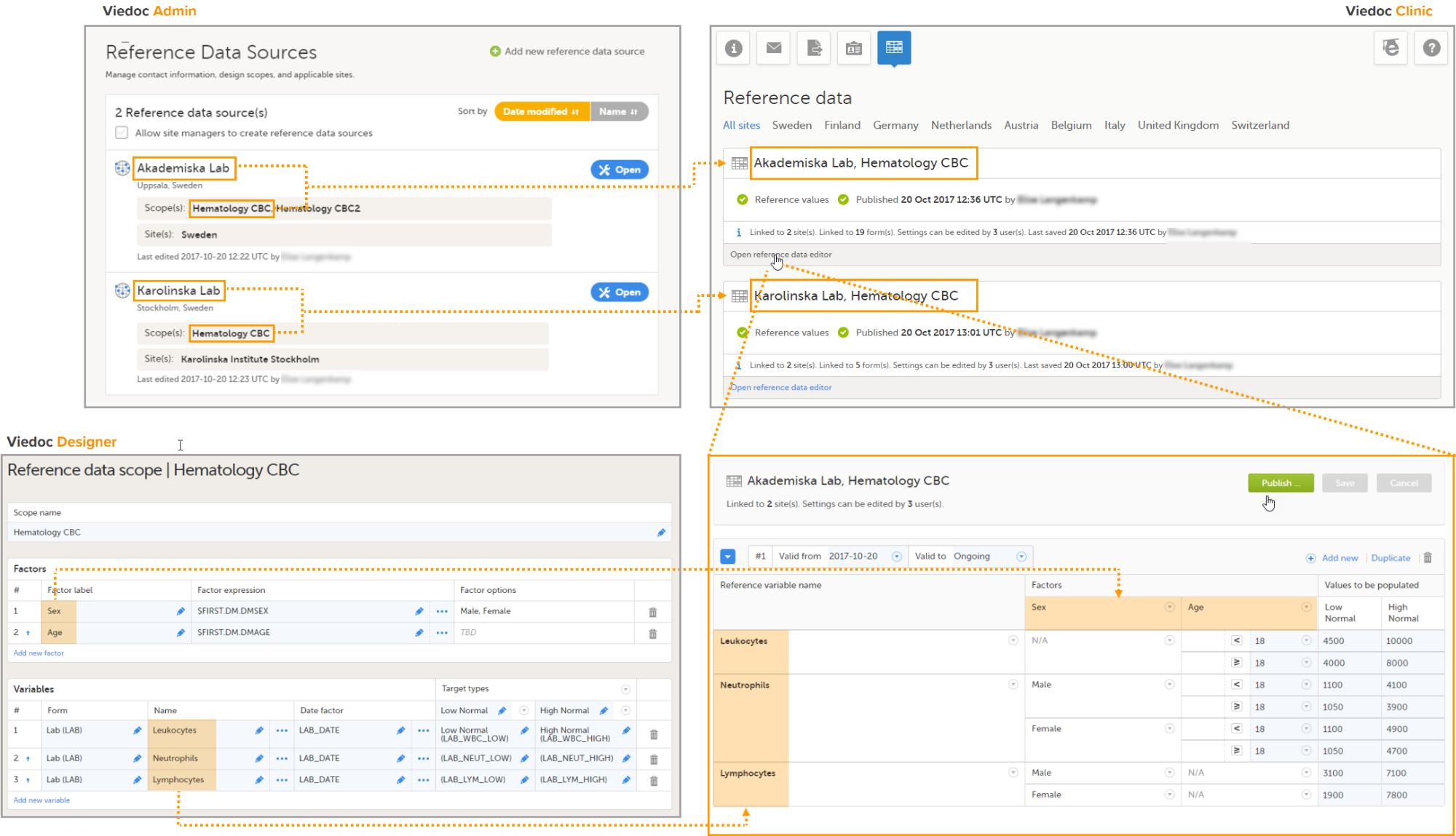
Configuring a reference data scope in Viedoc Designer
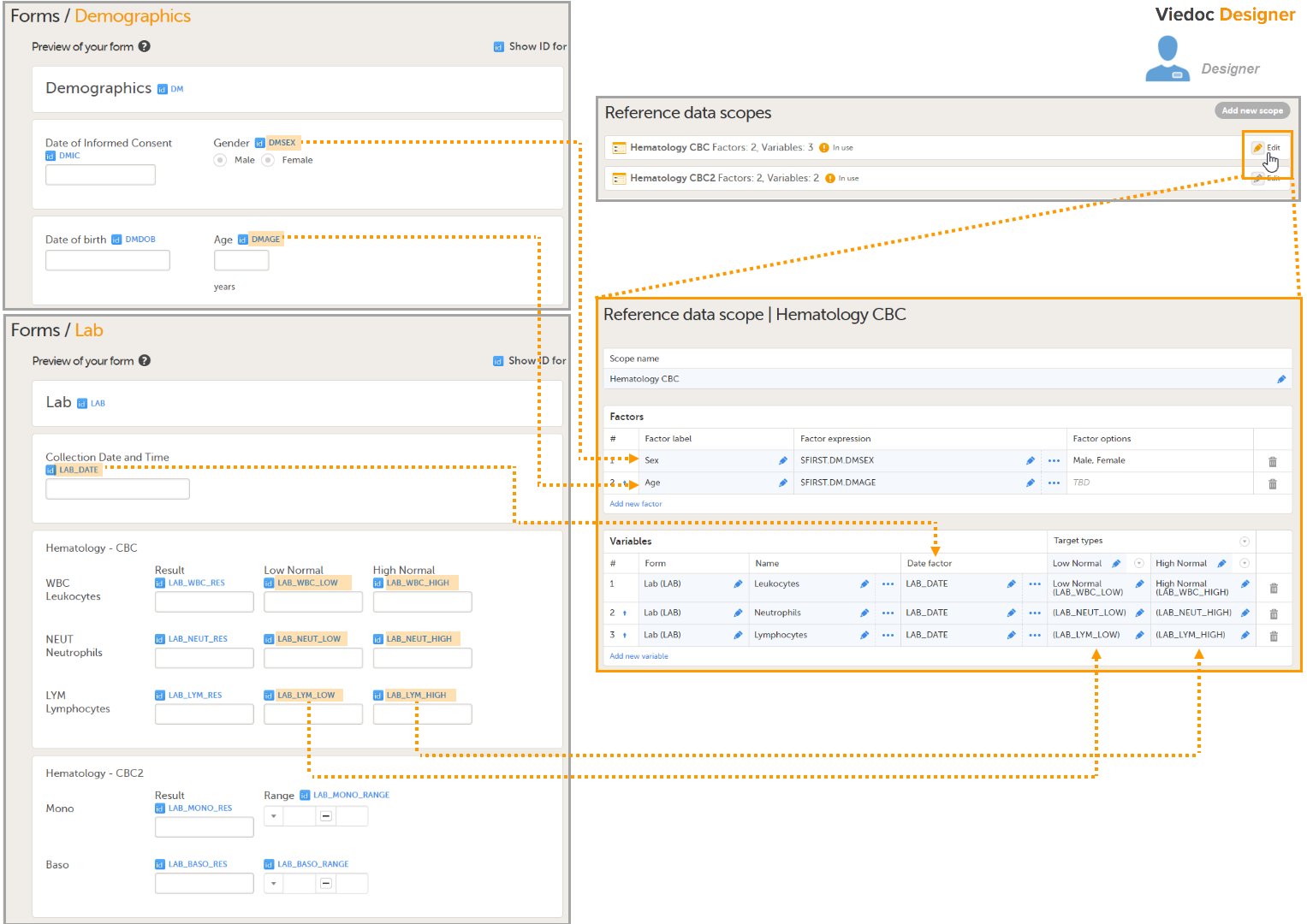
- In Viedoc Designer, create the form that will be auto-populated with reference values. In the example in the image, this form is the Lab form, and the items that will be populated with reference values are Low Normal, High Normal and Range.
- Set up a Reference data scope. The reference data scope defines a set of measurements that a reference data source (e.g., a lab) carries out, and the factors that might affect these data. In this example, the reference data scope Hematology CBC was set up, with:
- Sex and Age as Factors - because these are the factors that the respective reference values might depend on.
- Leukocytes, Lymphocytes and Neutrophils as Variables - because these are the parameters that are going to be measured. For each variable, we set the date factor (the date on which the reference values to be populated are based) to the LAB_DATE item in the Lab form. We also set up two target types that correspond to the Low Normal and High Normal items in the Lab form.
- Publish the Global design settings, so that the defined reference data scope will become available in Viedoc Admin and Viedoc Clinic.
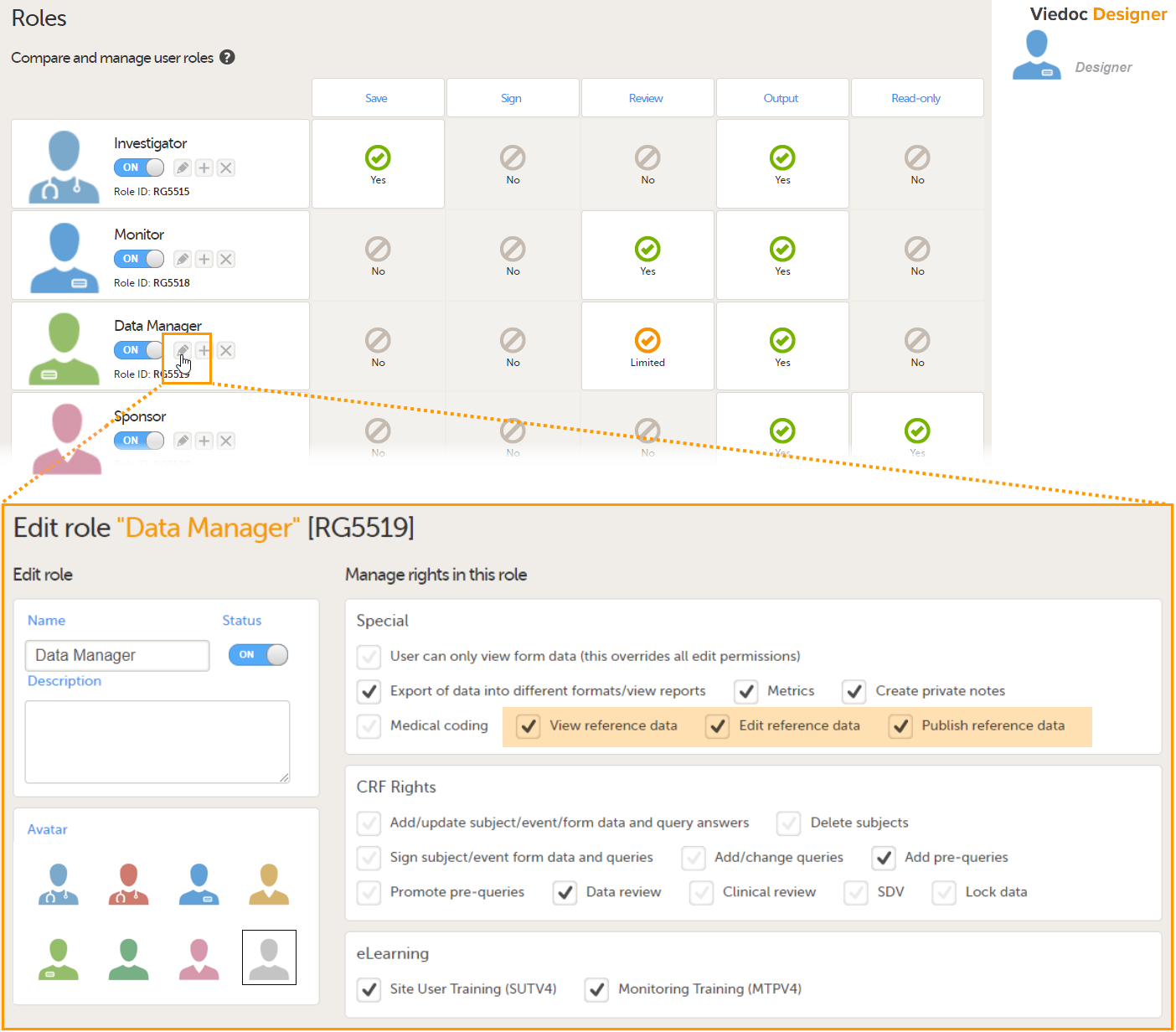
- Create one or more clinic roles that have permission to perform one or more of the following actions:
- View reference data - allows the user to see the existing reference data in read only mode in Viedoc Clinic. When enabling this option the following two options become available:
- Edit reference data - allows the user to edit and save reference data.
- Publish reference data - allows the user to publish the reference data values, so that the values will become available for the forms in Viedoc Clinic.
Note! You need to have at least one clinic role with permission to edit reference data and one clinic role with permission to publish reference data. This does not have to be the same role.
For more detailed instruction, see Configuring reference data scopes, and Configuring roles in Viedoc Designer.
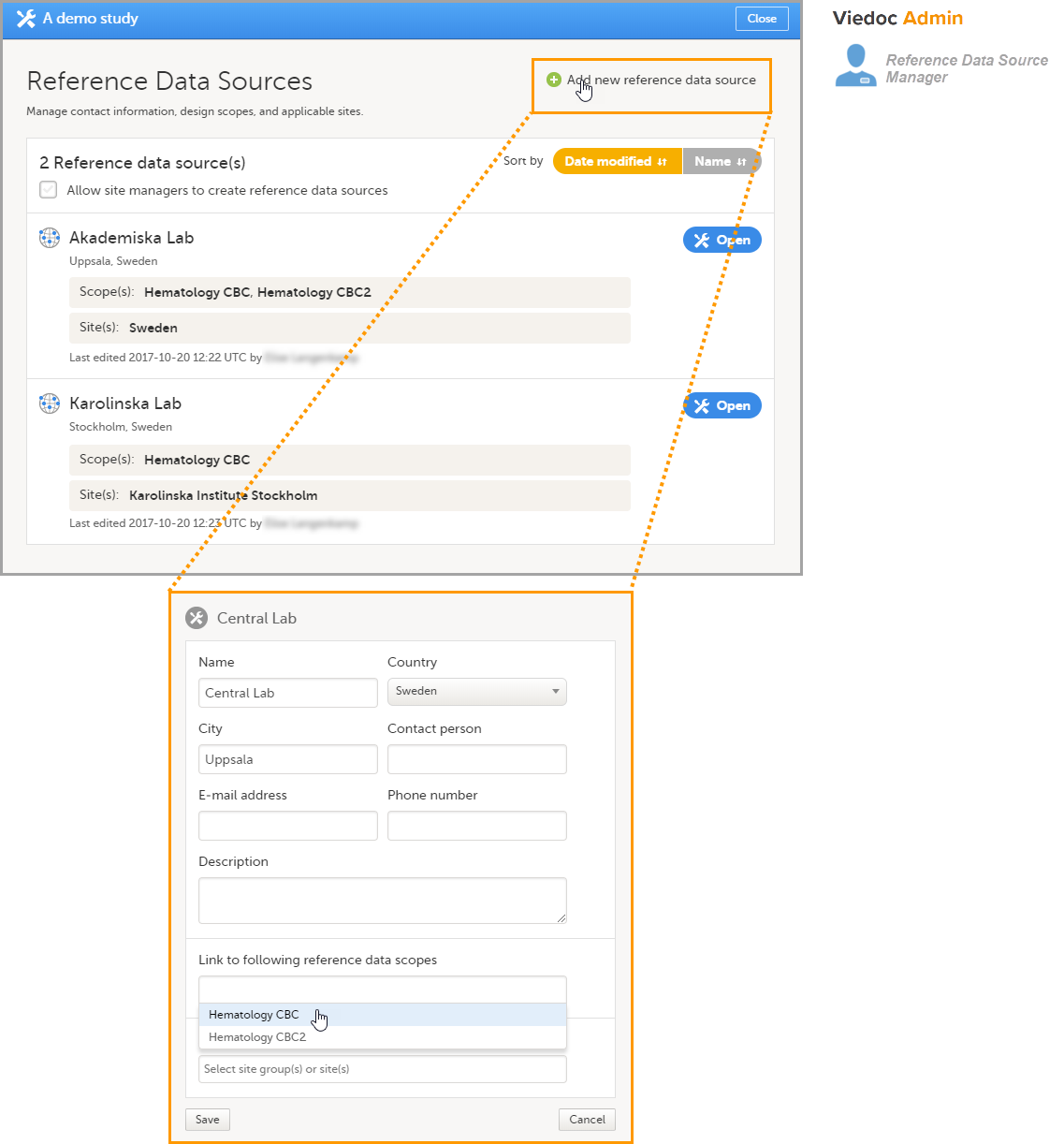
Adding a reference data source in Viedoc Admin
In Viedoc Admin, open the Reference data source(s) window and add the reference data sources (the labs or institutes that will provide the reference data). Link the reference data source to the reference data scopes and to the sites for which they should be used.
For more detailed instruction, see Managing reference data sources in Viedoc Admin.
In this example, we have defined two reference data sources: Akademiska Lab and Karolinska Lab. The Akademiska Lab is linked to two scopes: Hematology CBC and Hematology CBC2. It is also linked to the system site group Sweden (all production sites in Sweden). The Karolinska Lab is only linked to the scope Hematology CBC, and to the site Karolinska Institute Stockholm.
For each of the defined reference data source-scope combinations, reference data value sets will become available in Viedoc Clinic.
Entering reference values in Viedoc Clinic
- In Viedoc Clinic, on the landing page, click the reference data icon. A list of all reference data source-scope combinations is displayed.
- Click Open reference data editor to open the reference data editor. In this example, we enter the values for the Akademiska Lab, Hematology U source-scope combination.
- Select the time period the values are valid.
- Select the factors to include. In this example, both Age and Sex are included, yet not used for all three variables. We set sex to N/A (not applicable) for the variable Leukocytes, and age to N/A for the variable Lymphocytes.
- Select the factor options to include, and/or define the range. In this example, we include Male and Female as factor options for the factor Sex, and we specify <18 and ≥18 as ranges for the factor Age.
- Enter the reference values.
- Click Save to save the reference values.
- Click Publish to publish the reference values. Publishing will make the reference values available for autopopulation to the subject forms.
For more detailed instruction, see Working with reference data in Viedoc Clinic.
Auto-population of reference data to the subject forms
- Open the form to which the reference data will be populated, in this example Lab. Viedoc automatically identifies forms that have items that belong to reference data scopes, and displays a section in which the source for the reference data can be selected: Link the scope with the reference data source that provided the test results.
- For each scope, select the reference data source that provided the reference data from the drop-down list. In this example we select Akademiska Lab for the scope Hematology CBC.
- Set the Collection date and time.
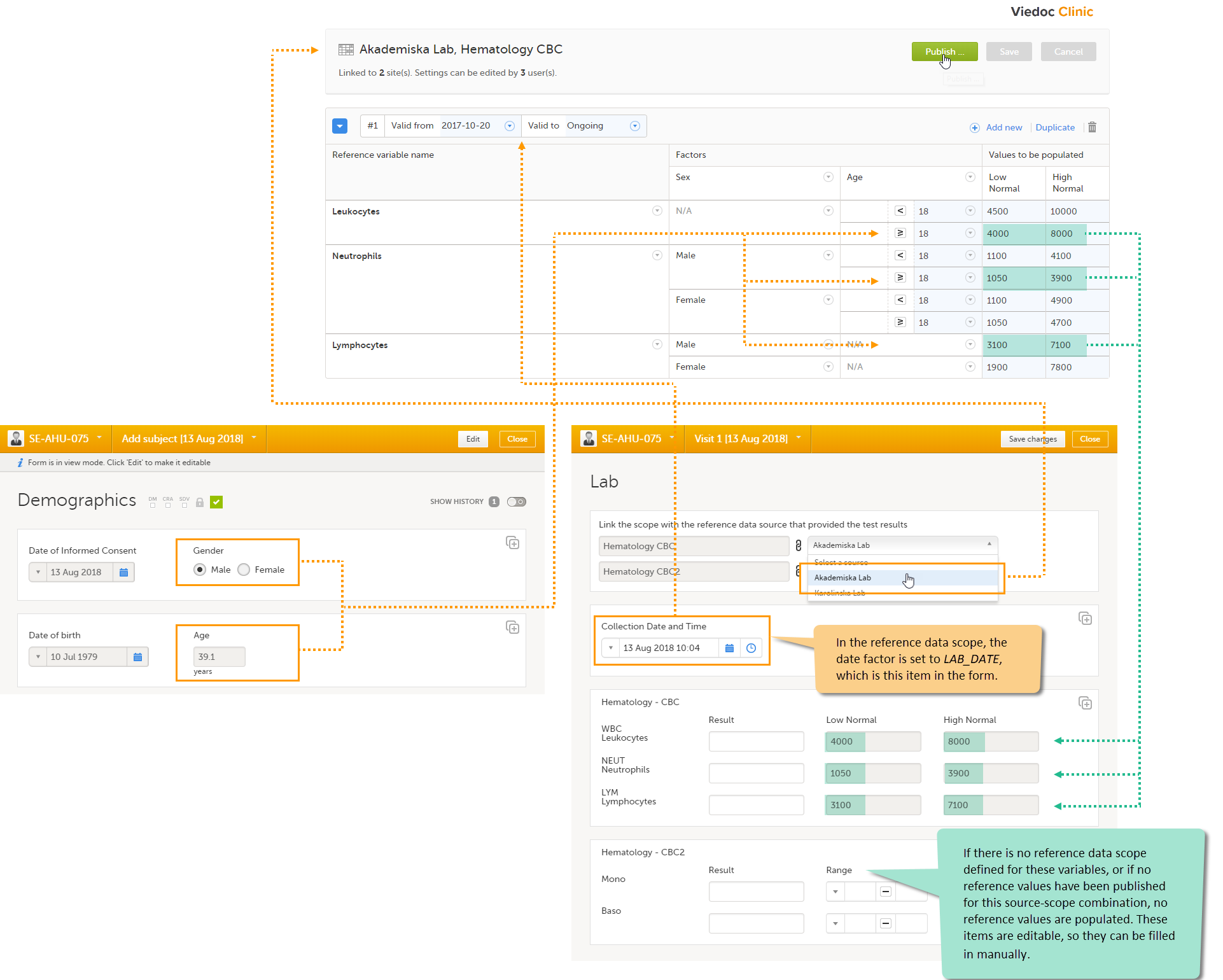
The system verifies:
- which date factor has been defined in the reference data scope (so on which date the reference values should be based), and whether this date lies within the time period that the reference values are valid. In this example, the date factor is set to the item LAB_DATE, which has the value 13 Aug 2018 10:04. This date lies within the time period #1 that the reference values of the source-scope combination Hematology CBC-Akademiska Lab is valid.
- what the factors are, in this example the gender (male) and the age (39, thus ≥18) of the subject. This information is taken from the Demographics form.
If the date matches the validity of the reference values, the system auto-populates the relevant reference values to the subject form, based on the defined factors.
If you do not select a reference data source, no values will be automatically populated. The items are editable so that they can be filled in manually. Similarly, if no scope is defined (as for the Mono and Baso items in the form), or if no reference values are entered for that specific source-scope combination or for that specific date, the items remain empty and can be filled in manually.
For more detailed instruction, see Working with reference data in Viedoc Clinic.
