Submitting and recalling booklets, AE reports and forms.
Introduction
Viedoc Post Marketing Surveillance (PMS) offers support for collecting data in booklets, allowing data to be collected during a specific time period rather than a specific event date, and support for sending and receiving booklets back and forth between site and sponsor. This process of handling booklets in a Japanese PMS study is known as the Kaifu process. For an overview of this process, see Overview of the submit-receive-return process.
In the submit-receive-return (Kaifu) process, the clinic user chooses when to share data with the sponsor and the sponsor side user chooses when to receive the data. One of the important characteristics of this process is that the sponsor side user does not have access to any data entered in a booklet, until the booklet has been submitted by the clinic, and a receive action has been actively performed by the user at the sponsor side.
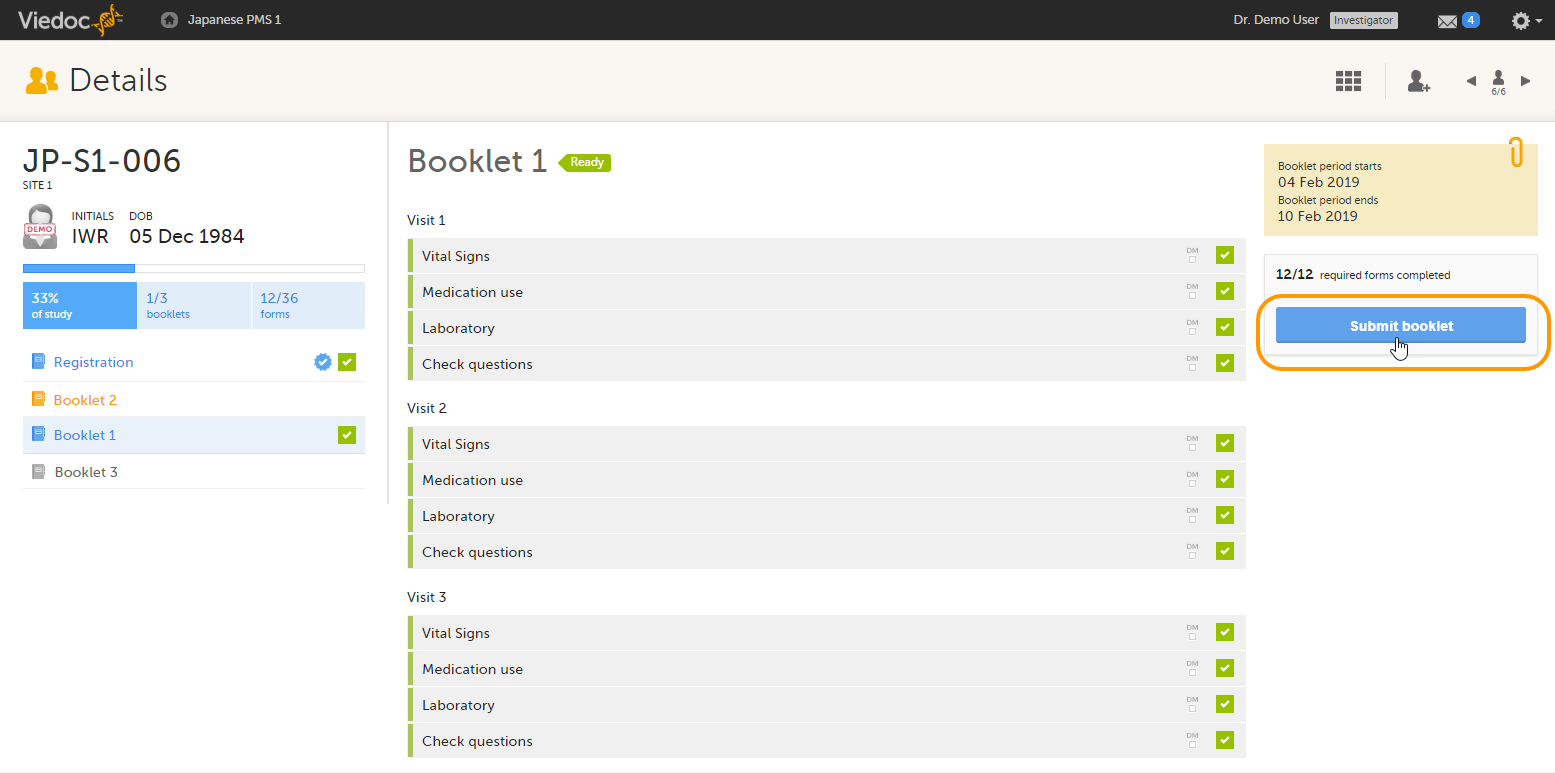
Booklets can only be submitted to the sponsor when all events and forms in the booklet are completed, see Submitting a booklet for instructions.
There is an exception to this rule, which depends on your study configuration. It is possible that some forms (for example, but not limited to, Adverse Events) can be managed, that is, submitted/recalled individually. When these forms are saved, a warning message is displayed and a Manage link is available. For more information, see Managing individual forms.
Note! As soon as an AE is added, the system displays a warning message at the top of the booklet to facilitate the timely submission of AEs.
The status of the booklet is displayed on the subject Details page. You can select to display the complete history of submitting-receiving-returning the booklet by clicking Show history. You can select to display only the current status of the booklet by clicking Hide history.
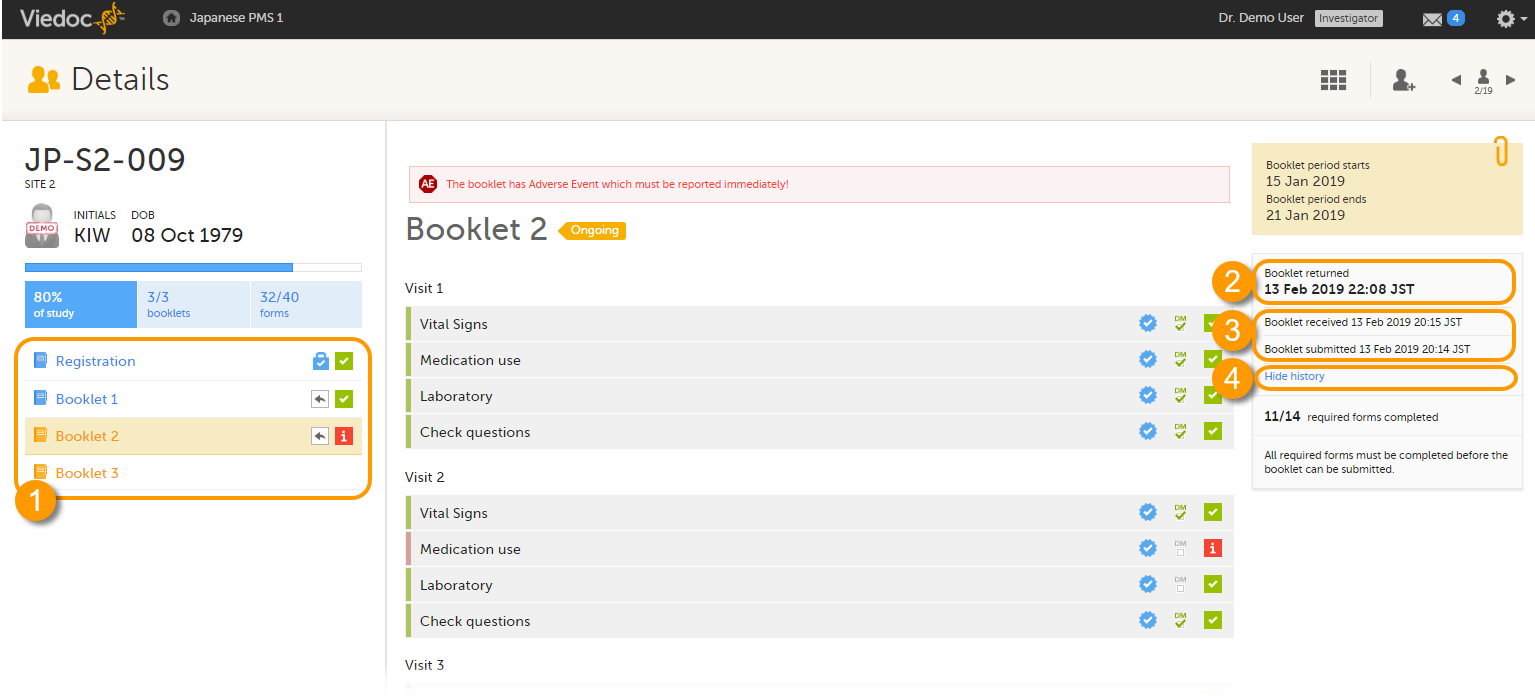
1. Select a booklet to work with.
2. Current status of the selected booklet.
3. History of the selected booklet.
4. Show/hide history.
Submitting a booklet
Note! Booklets can only be submitted to the sponsor when all events and forms in the booklet are completed. It however is possible to submit an AE or another form separately, (depending on your study configuration), before all data in the booklet is completed. For information on how to submit an AE or another form, see Managing individual forms.
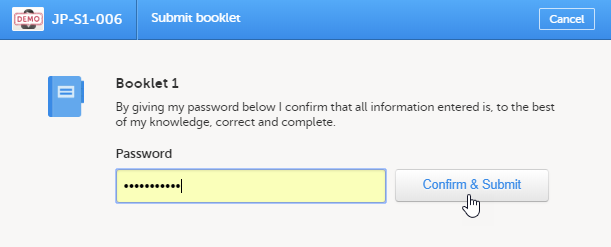
To submit a booklet:
| 1 |
Click Submit booklet. |
| 2 |
Enter your password and click Confirm & Submit. |
The booklet status changes into submitted and the Recall submitted booklet icon appears.
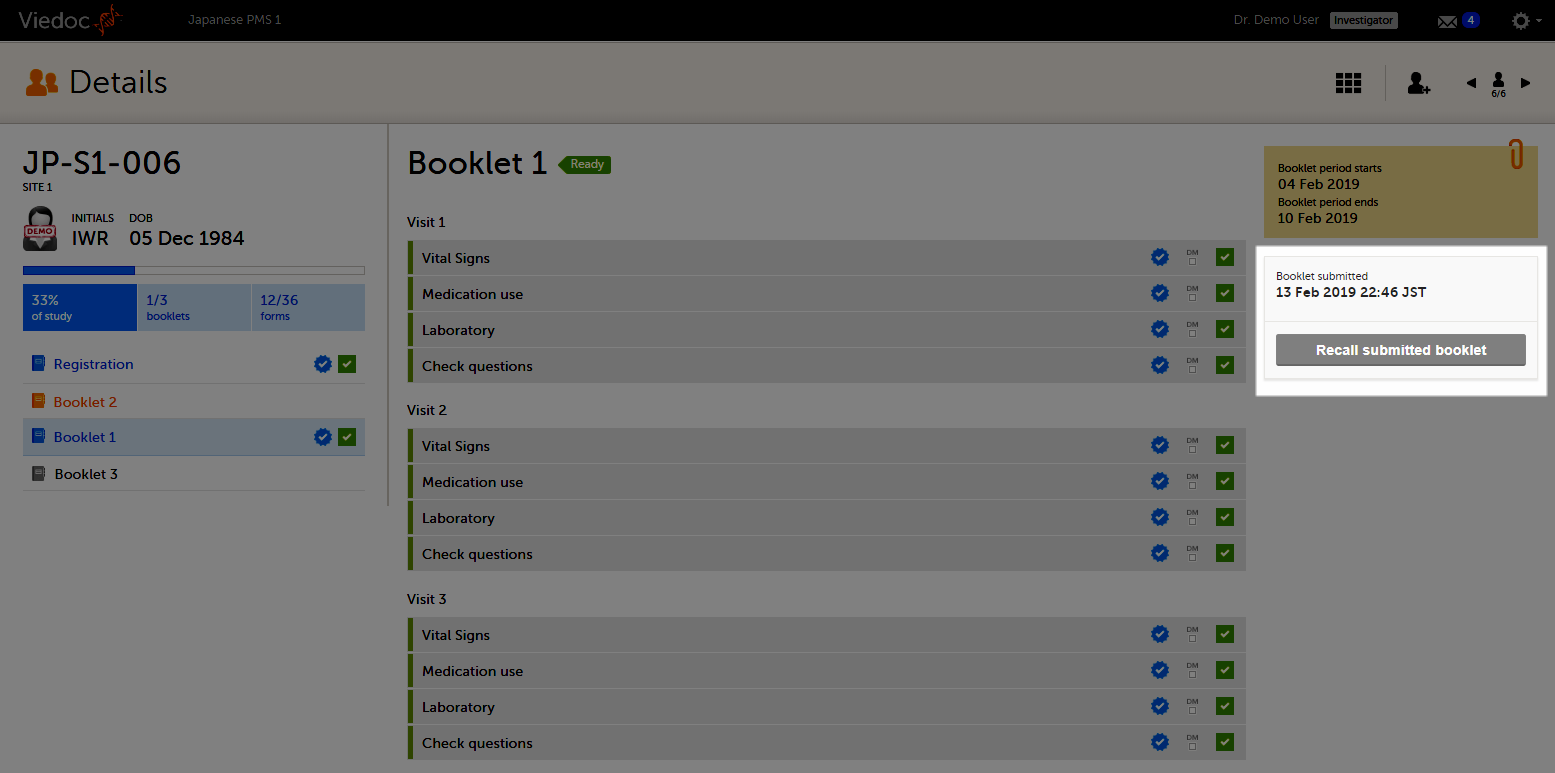
On the booklet overview, the booklet receives the following blue checkmark icon, indicating that the booklet has been submitted. Each form in the booklet receives the same icon indicating that all data in the form is signed.
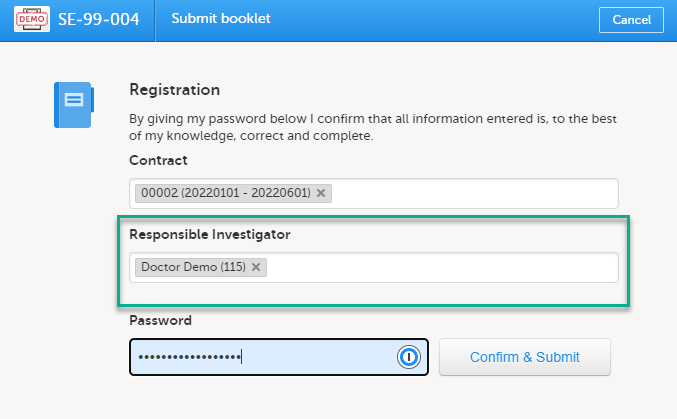
Linking a Responsible Investigator to a booklet submission
If the option Require Responsible Investigator for booklet submission is enabled for your study, the booklet can be linked to a Responsible Investigator at the time the booklet is submitted. This is for full booklet submissions only and is not available for partial (AE) submits.
When first submitting the registration booklet, and all of the succeeding booklets for that subject, the current user is automatically set as the Responsible Investigator.
Note! If the Responsible Investigator that was assigned when the registration booklet was submitted can no longer access the site, their user name is still displayed, and the following error message is shown:
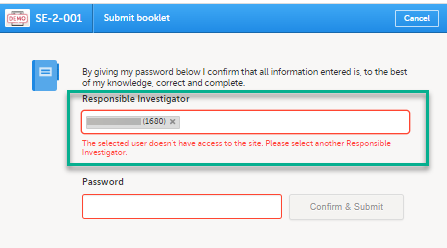
A different Responsible Investigator must be selected to submit the next booklet. If you select another user as a Responsible Investigator, this will be overridden. All clinic side users with access to the selected site (and who have accepted access) can be selected. The user is displayed by the User name (internal user ID). If the booklet was returned, the Responsible Investigator is the same user that was selected for the submission. This can, however, be changed to another Responsible Investigator.
Note! For the registration booklet, the applicable contract is selected by default, by matching the event date to the validity period of the contract for the registration booklet.
Linking a contract to a booklet submission
If the option Require Contract for booklet submission is selected, the booklet can be linked to a contract at the time the booklet is submitted. This is for full booklet submissions only and is not available for partial (AE) submits.
For the registration booklet, you can select a contract when submitting as shown in the image below:
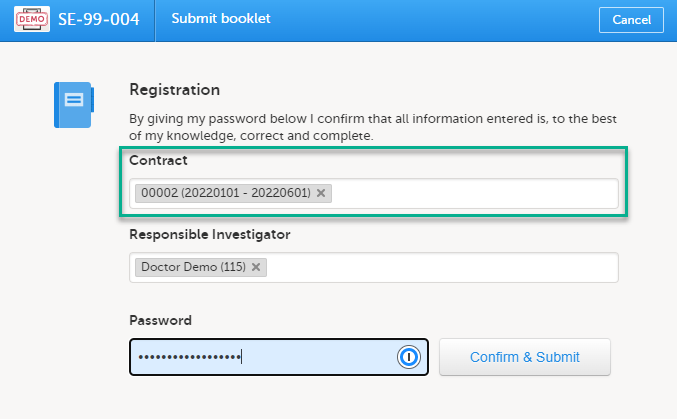
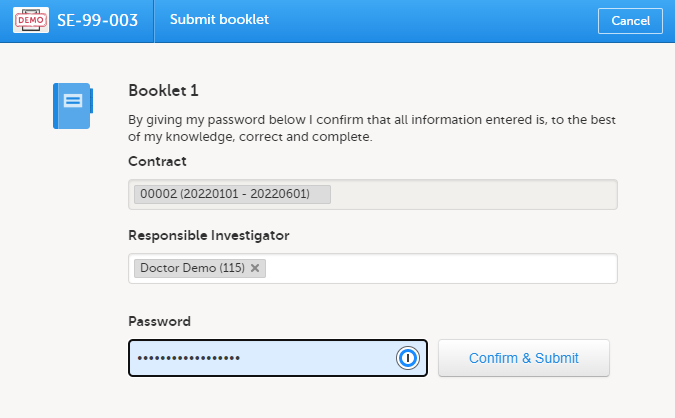
For all succeeding booklets for the same subject, the contract is set to the same contract as used for the registration booklet and cannot be changed (that is, read-only for all booklets except the registration booklet).
If no contracts were added for the site, so that the Contract list is empty when submitting the booklet, the following message is displayed in the Submit booklet window:
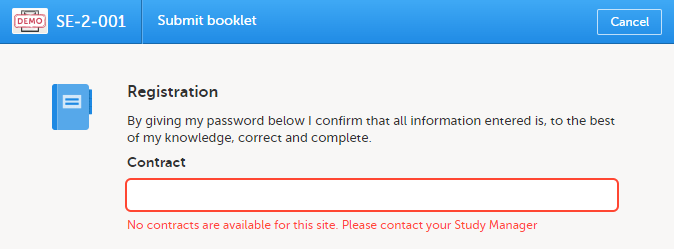
You can change the selected default contract to any other contract that exists for the site. The existing contracts for the site are listed in chronological order of their validity period.
If the booklet was returned, the contract is the same as was previously selected. This can, however, be changed to any of the existing contracts for the site.
| Important! If a subsequent booklet is submitted before the registration booklet, the default selection applies for both Contract (that is, by default the contract that matches the event date of the booklet, and possible to be changed to any other existing contract for the site) and Responsible Investigator. |
Recalling a booklet
Note! A submitted booklet can only be recalled if it has not yet been received by the sponsor.
To recall a booklet, click Recall submitted booklet.
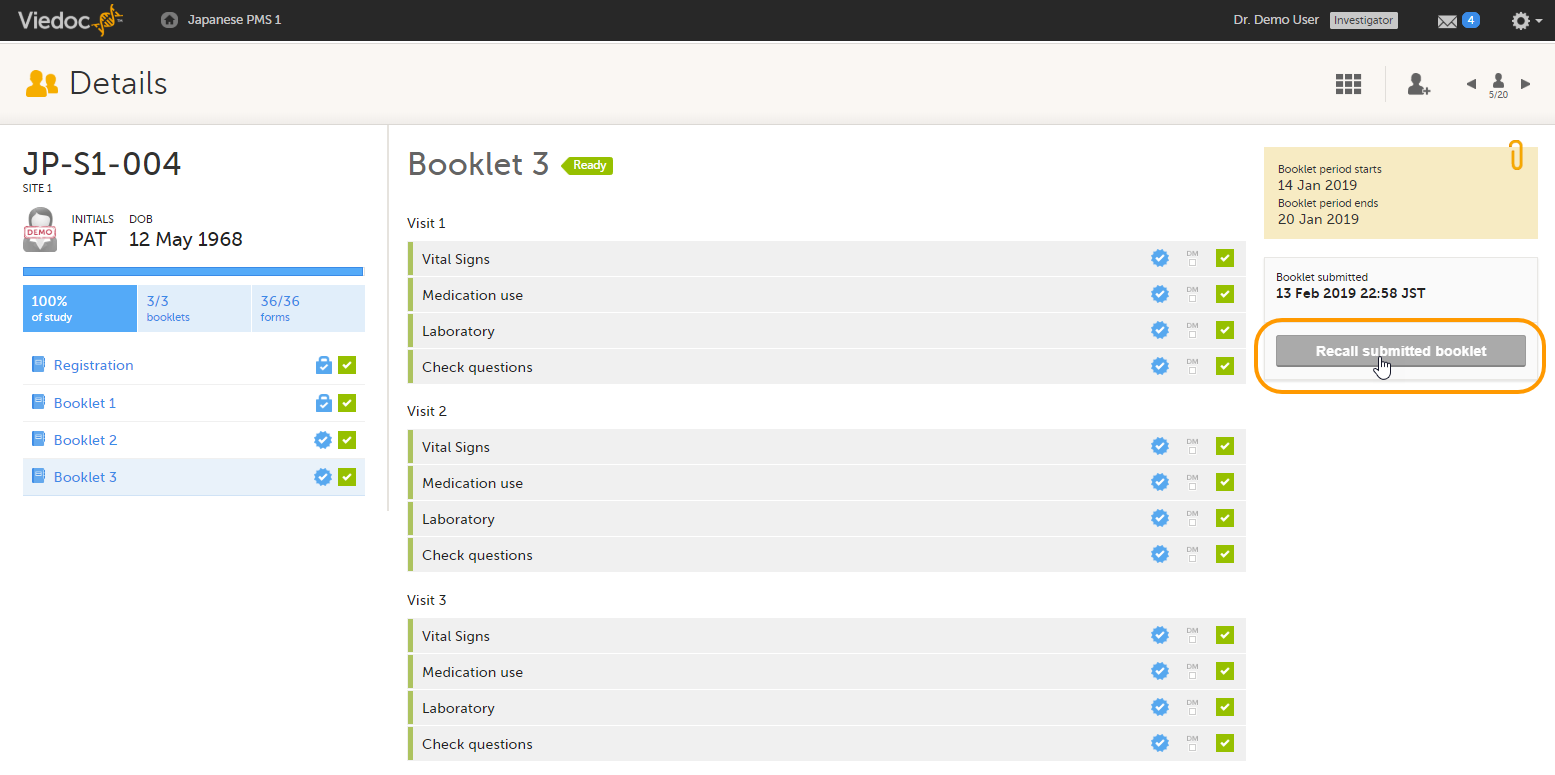
The booklet status changes into recalled and the Submit booklet icon appears. The Submit booklet icon disappears as soon as data in one of the forms in the booklet is deleted.
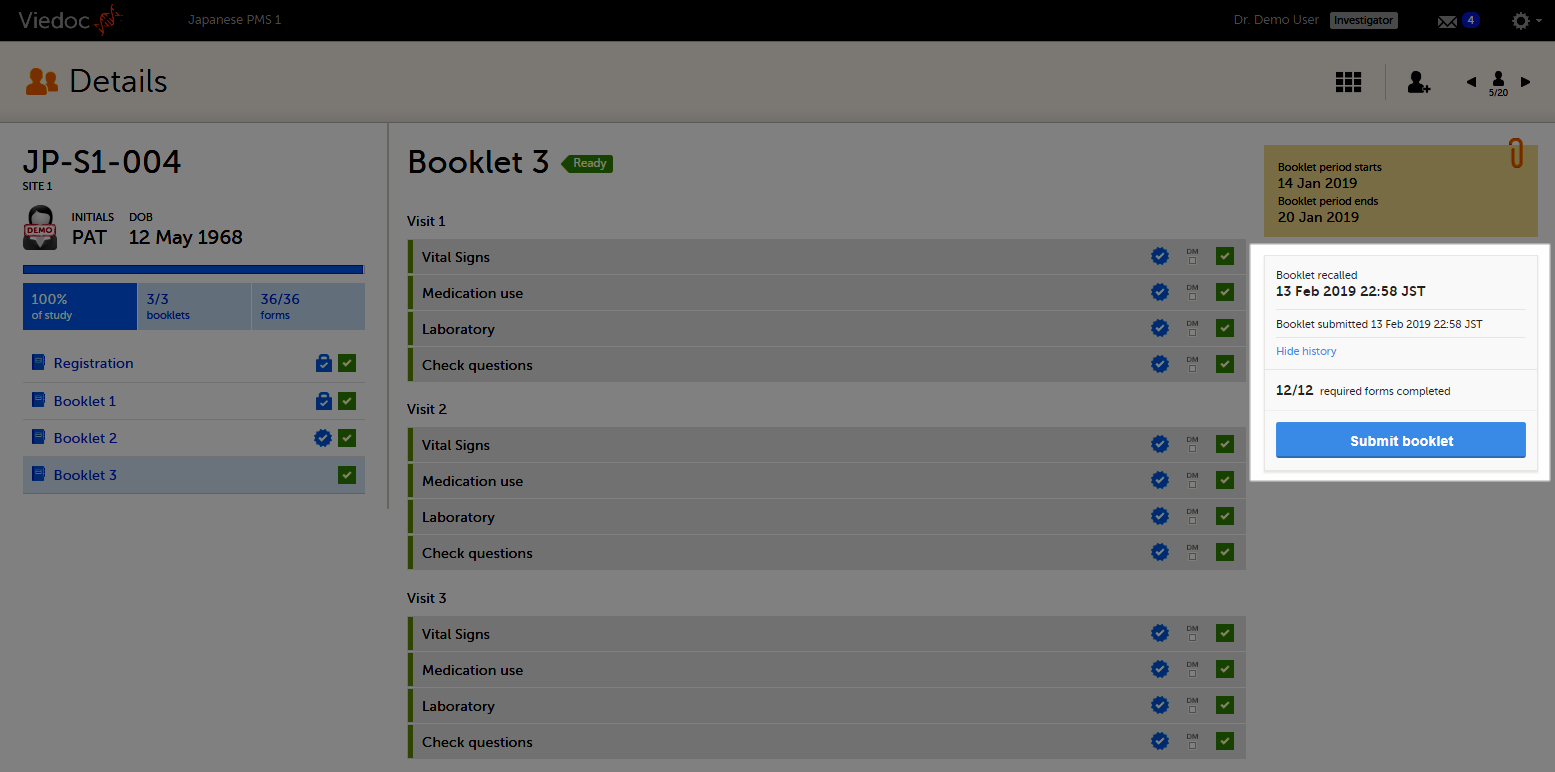
On the booklet overview, the booklet receives the blue checkmark icon, indicating that the booklet has been submitted.The icon disappears when the booklet is recalled. The blue checkmark icons on each form in the booklet remain, even if the booklet is recalled. They disappear when data in a form is changed, and re-appear upon re-submission of the booklet.
Managing individual forms
Overview
Depending on your study configuration, some forms, such as Adverse Events or other forms, can be submitted and recalled individually. This applies even if the booklet is not yet completed.
Note! When all forms within a booklet have been submitted individually, it is still possible to submit the booklet. The booklet status must be either initiated, recalled or returned and all of the forms must be filled in and without issues.
There are different icons, warning messages and dialogs shown in Viedoc Clinic, depending on your study configuration regarding which forms can be submitted individually.
Icons and warning messages
The icons displayed on the form are as described below:
| For forms marked as Adverse Events, the AE icon is shown. | |
|
For other forms, a red exclamation mark icon is shown. If the study has this configuration, the same AE icon is shown on AE forms, as long as the AE form has been configured as an AE in the study design. |
The warning messages are as shown below:
- If your study is configured to allow submitting other forms individually, the warning message shown at the top of the booklet is as configured in the study design.
- If your study is configured to allow submitting only AE forms individually, the following warning message is shown:

| When submitting an AE separately as an AE report: | When submitting other forms individually: |
|
When an AE is registered, a warning message appears at the top of the booklet. The AE has the status Not submitted, and a red AE icon appears on the form: 
|
When another type of form is registered, a warning message appears at the top of the booklet. The form has the status |
Submitting forms
Note! If your study is configured to allow a form to be submitted individually only if based on a condition, and that condition is not fulfilled, a message is displayed in the submit window and you cannot submit the form.
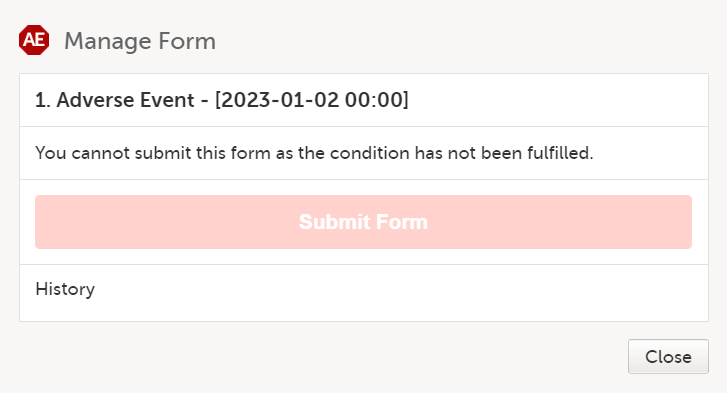
To submit a form within a booklet:
| 1 |
On the relevant form, select Manage. 
|
||
| 2 |
A window appears. Depending on the study configuration and the form to be submitted, the text is as shown below: Manage Form/Manage Adverse Event. Select Submit Form or Submit Adverse Event: 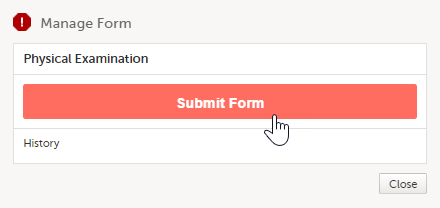 
|
||
| 3 |
A window appears asking you to confirm with your password. Enter your password to confirm submission of the form and select Confirm & Submit. 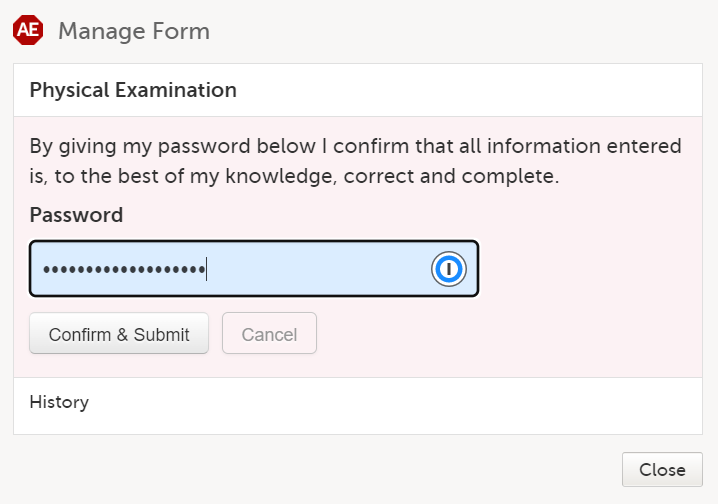 
|
||
|
The status of the form/AE form changes from Not submitted to Form submitted/Adverse Event submitted + date of submission and the warning message at the top of the booklet disappears. 
|
|||
|
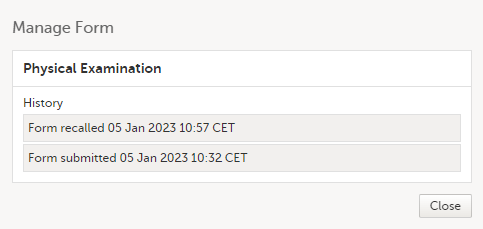
The complete history of the form submission/recall/receive is shown in the History in the Manage Form/Manage Adverse Event window that appears when selecting Manage. 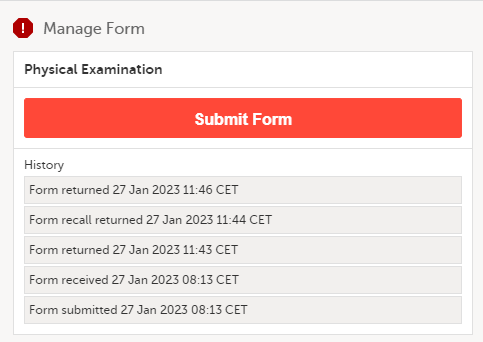

|
|||
Recalling a form
Note! A submitted form or an AE report can only be recalled by clinic users with permission to submit forms if it has not yet been received by the sponsor.
To recall a form:
| 1 |
On the relevant form, select Manage. 
A window appears. Depending on the form to be recalled, the dialog is as shown below: |
|
| 2 |
For Adverse Event forms, select Recall Adverse Event: 
|
For other forms, select Recall Form: 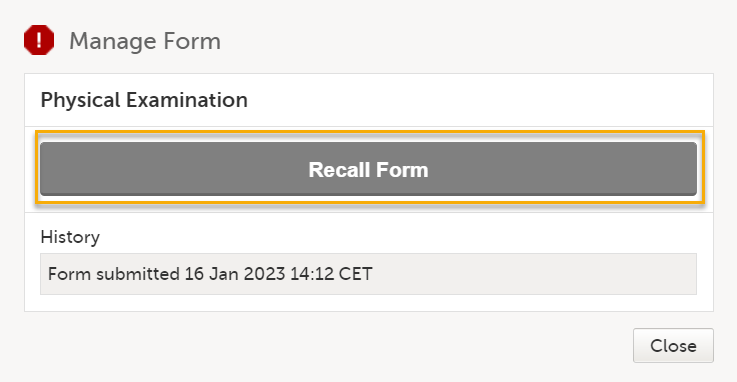
|
| 3 |
The status of the Form/Adverse Event changes from Form name/Adverse Event submitted + date of submission to Not submitted Form name/Adverse Event recalled + date of recall, and the warning message at the top of the booklet re-appears. The form can now be edited. 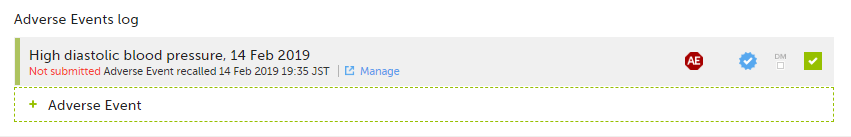
The complete history of the form submission/recall/receive is shown in the history in the Manage Form/Manage Adverse Event window that appears when selecting Manage. |
|
Managing linking forms and linked forms
Overview
Depending on your study configuration:
- You can submit a form that links to another form in order to add complementary data to a submitted form. For example, link an AE form that links to a Prior and Concomitant Medications form.
- Either the linking form (in the example above, the AE form), or the linked form (in the example above the Prior and Concomitant Medications form) can be submitted and recalled individually or in a booklet submit. For more information about submitting individual forms, see Managing individual forms.
Note! The linking form (for example an AE), can be submitted only when the linked form (for example, Prior and Concomitant Medications) is filled in and without issues. If a linked form has issues the following message is shown:

Submitting linked forms
When a linking form (for example an AE) is linked to another form (for example the Prior and Concomitant Medications form), and the AE (linking) form is submitted individually, a read-only copy of the linked form (the Prior and Concomitant Medications form) is submitted to the sponsor side (if it is not already available on the sponsor side).
Note! The linked form has the status read-only on the clinic side, until the linking form together with the linked form copy is either received by the sponsor side, or is recalled by the clinic side.
The linking form and the linked form can be in the same booklet or in different booklets.
To submit a form linked to another form:
| 1 |
On the relevant form, select Manage. 
|
| 2 |
A window appears with the text as shown below: 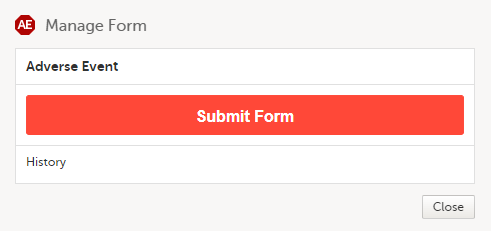
Select Submit Form. |
| 3 |
A window appears asking you to confirm with your password. Enter your password to confirm submission of the form and select Confirm & Submit. 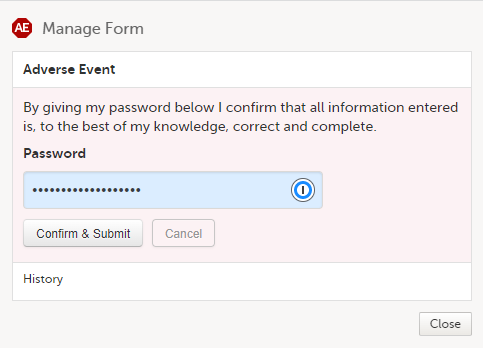
The status of the form changes from Not submitted to Form submitted + date of submission and the warning message at the top of the booklet disappears. 
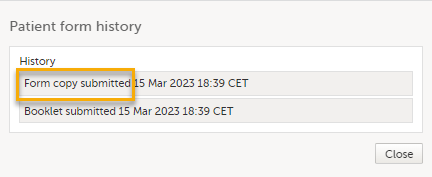
At the same time, a read-only copy of the linked form is submitted. When a read-only copy of the linked form is submitted, in the Manage window that appears when selecting Manage, the History is updated with Form copy submitted. 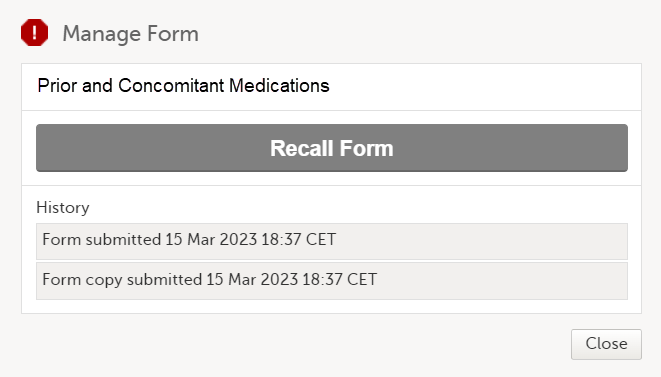
When the linked form copy is submitted, it can not be edited until the form has been received on the |
Linked form history
When submitting a linked form, depending on how your study is configured, the links shown on the forms are as below:
If the linked form is also a form that can be submitted individually, the Manage link is shown:

For forms that can be submitted and recalled individually, the complete history of the linked form submission/recall/receive is shown in the Manage window that appears when selecting Manage: When a read-only copy of the linked form is submitted, the History is updated with Form copy submitted.

Note! If the linked form cannot be submitted/recalled individually:
- The Linked form history link is shown, as a read-only copy of the form is submitted and no submit/recall actions can be performed.
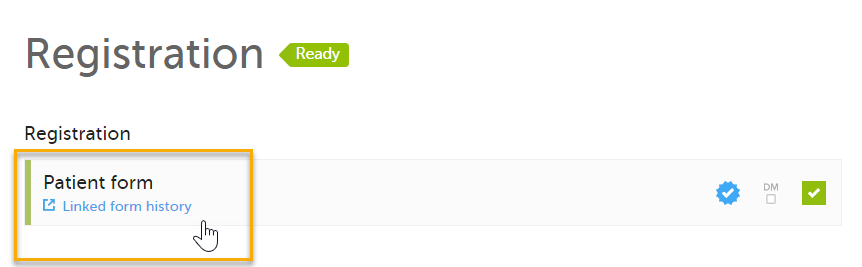
- The complete history of the linked form submission/recall/receive actions in the booklet, including the form copy actions, is shown in the History window that appears when selecting Linked form history.
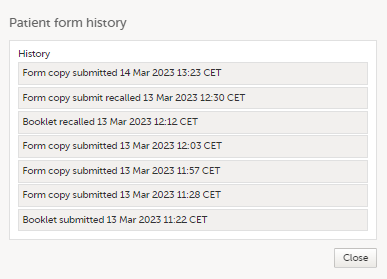
- When a read-only copy of the linked form is submitted, the Linked form history is updated with Form copy submitted.
Submitting a booklet with linked forms
To submit a booklet containing linked forms, follow the steps for Submitting a booklet.
A booklet containing a linking form (for example an AE), can be submitted only when the linked form (for example, Prior and Concomitant Medications) and all other forms in the booklet are filled in and without issues.
If any of the forms have issues, the following message is displayed:

Note! If a booklet contains a linking form and the linked form is part of that booklet when the booklet is submitted, the linked form is not submitted as a read-only copy but as a standard booklet submit. For more information about read-only copies of forms, see Submitting linked forms.
Recalling linking forms
A linking form can be recalled either as an individual form or as part of a booklet, and the linked form can be within the same booklet or in a different booklet.
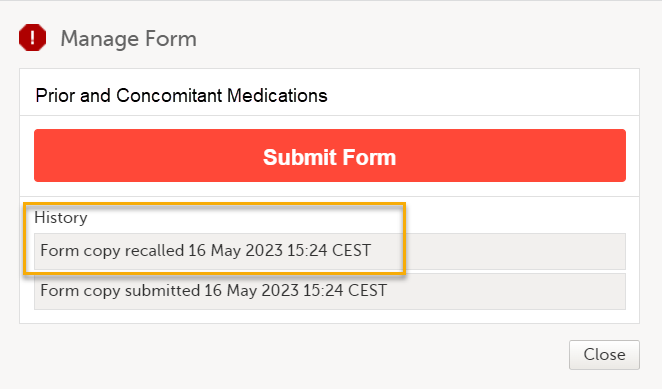
Note! When the linking form is recalled, a linked form that has been submitted as a read-only copy is also recalled, and the status of the linked form changes from Form name/Adverse Event submitted + date of submission to Not submitted + Form copy recalled + date of recall. The linked form can now be edited.
Updates to partial submit settings in new study design versions
The following applies to updates to partial submit settings in a new study design:
When a new study design version is assigned, with a form that is configured to be submitted individually, all of the existing (saved) forms can be submitted individually and the warning message is shown.
When a new study design version is assigned, where it is no longer possible to individually submit a form, the following applies for the existing (saved) forms:
- Clinic users can still view the previously saved forms but cannot submit forms.
- There are no warning messages displayed on the subject details page for that form.
- The Not submitted message on the subject details page is not shown.
- The Manage link can still be selected to show the complete history of the form's submit-receive-return actions, if the form was individually submitted, but not if the form was part of a booklet submission.

 A confirmation window opens.
A confirmation window opens.