Review Status
Review status
You can sort the data to focus on the review status by country, site, event, subject, or form, with the following columns showing, respectively:
by Country
- Study, Country
- Percentage (%): CR, DM, SDV, Sign, Lock
- Count (n/N): CR, DM, SDV, Sign, Lock
- # of subjects
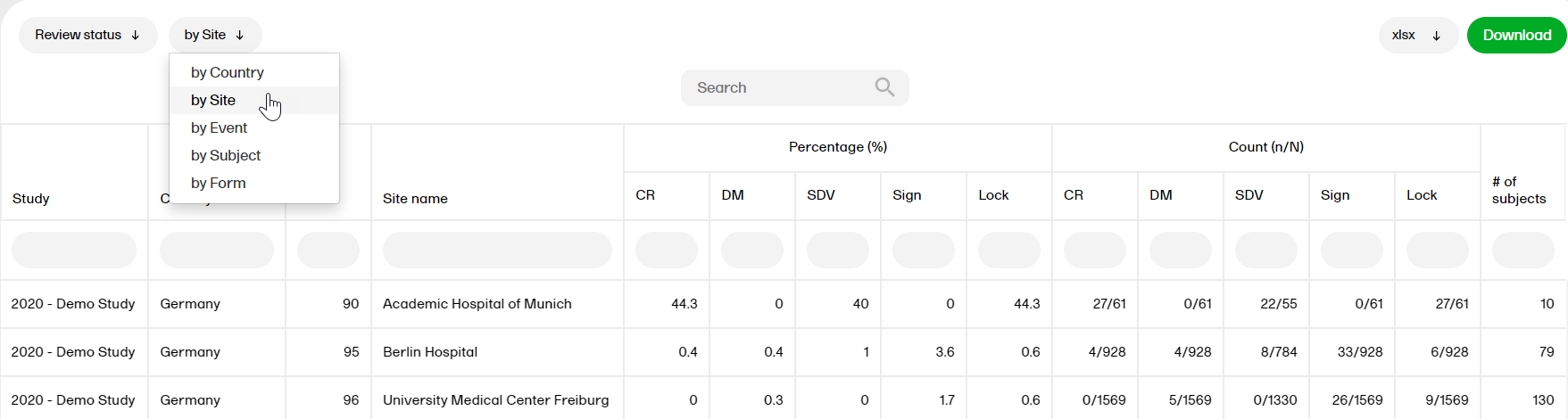
by Site
- Study, Country, Site Code, Site Name
- Percentage (%): CR, DM, SDV, Sign, Lock
- Count (n/N): CR, DM, SDV, Sign, Lock
- # of subjects
by Event
- Study, Country, Site Code, Site Name, Event
- Percentage (%): CR, DM, SDV, Sign, Lock
- Count (n/N): CR, DM, SDV, Sign, Lock
- # of subjects
Rows in the report are ordered according to the events of the latest effective design.
by Subject
- Study, Country, Site Code, Site Name, Subject
- Percentage (%): CR, DM, SDV, Sign, Lock
- Count (n/N): CR, DM, SDV, Sign, Lock
by Form
- Study, Country, Site Code, Site Name, Subject, Event, Event Sequence, Event Date, Form, Form sequence, Clinical Review By, Clinical Review Date
Rows in the report are ordered according to the events of the latest effective design.
n/N = number of forms reviewed for a specific status / total number of forms it is possible to review for a specific status.
Notes!
- Event Date is not included in the Form Status, but it is included in the Review Status. This means that N in the review status is greater than the number of triggered forms, for example, in the form status.
- For SDV, N is the total number of forms where SDV is possible. The N for other statuses counts the total number of forms as they apply to all forms.
- Forms that have partial items selected for SDV will display as blank, instead of Not required, in case SDV is pending.
CR Percentage (%)
| Sub report | Description |
|---|---|
| Country | The percentage of the initiated forms that have been reviewed of the initiated forms that can be reviewed. (Hidden forms are not considered). |
| Site | |
| Event | |
| Subject |
DM Percentage (%)
| Sub report | Description |
|---|---|
| Country | The percentage of initiated forms that have been data reviewed of the initiated forms that can be data reviewed. (Hidden forms are not considered). |
| Site | |
| Event | |
| Subject |
SDV Percentage (%)
| Sub report | Description |
|---|---|
| Country | The percentage of initiated forms that have been source data verified of the initiated forms that can be source data verified. (Hidden forms are not considered). |
| Site | |
| Event | |
| Subject |
Sign Percentage (%)
| Sub report | Description |
|---|---|
| Country | The percentage of initiated forms that have been 'signed' of the initiated forms that can be 'signed'. (Hidden forms are not considered). |
| Site | |
| Event | |
| Subject |
Lock Percentage (%)
| Sub report | Description |
|---|---|
| Country | The percentage of initiated forms that have been 'locked' of the initiated forms that can be 'locked'. (Hidden forms are not considered). |
| Site | |
| Event | |
| Subject |
CR Count (n/N)
| Sub report | Description |
|---|---|
| Country | n = Clinical reviewed forms. N = The total number of initiated forms where CR is required (hidden forms are not considered for CR). |
| Site | |
| Event | |
| Subject |
Note! n/N = number of forms reviewed for a specific status / total number of forms it is possible to review for a specific status.
DM Count (n/N)
| Sub report | Description |
|---|---|
| Country | n = Data reviewed forms. N = The total number of initiated forms where DM is required (hidden forms are not considered for DM). |
| Site | |
| Event | |
| Subject |
SDV Count (n/N)
| Sub report | Description |
|---|---|
| Country | n = Source data verified forms. N = The total number of initiated forms where source data verification is required, either for the entire form or at least one item on the form. |
| Site | |
| Event | |
| Subject |
Sign Count (n/N)
| Sub report | Description |
|---|---|
| Country | n = Signed forms. N = The total number of initiated forms where Sign is required (hidden forms are not considered for Sign). |
| Site | |
| Event | |
| Subject |
Lock Count (n/N)
| Sub report | Description |
|---|---|
| Country | n = Locked forms. N = The total number of initiated forms where Lock is required (hidden forms are not considered for Lock). |
| Site | |
| Event | |
| Subject |
# of Subjects
| Sub report | Description |
|---|---|
| Country | The total number of subjects. |
|
Site |
|
| Event |
Reviewed Item
| Description | |
|---|---|
| Event | A reviewed item will display an event, if the review action was performed on the event date. |
| Form | A reviewed item will display a form, if the review action was performed at the form level. |
Clinical Review By
| Description |
|---|
| The Username and ID of the user who performed the clinical review. Note! In the case where the event date form is excluded from Viedoc Clinic, then in Viedoc Reports - Review Status report, the 'Clinical Review by' will be marked as "N/A" (not applicable) since the form cannot be signed. |
Clinical Review Date
| Description |
|---|
|
The date and time in UTC (Universal time coordinated) when the clinical review was performed. |
DM Review By
| Description |
|---|
|
The Username and ID of the user who performed the data review. Note! In the case where the event date form is excluded from Viedoc Clinic, then in Viedoc Reports - Review Status report, the 'DM Review by' will be marked as "N/A" (not applicable) since the form cannot be signed. |
SDV Review Date
| Description |
|---|
|
The date and time (UTC) when the data review was performed. |
SDV By
| Description |
|---|
|
The Username and ID of the user who performed the source data verification (SDV). Note! In the case where the event date form is excluded from Viedoc Clinic, then in Viedoc Reports - Review Status report, the 'SDV by' will be marked as "N/A" (not applicable) since the form cannot be signed. |
SDV Date
| Description |
|---|
|
The date and time (UTC) when the SDV was performed. |
Signed By
| Description |
|---|
|
The Username and ID of the user who signed the form. Note! In the case where the event date form is excluded from Viedoc Clinic, then in Viedoc Reports - Review Status report, the 'Signed by' will be marked as "N/A" (not applicable) since the form cannot be signed. |
Signed Date
| Description |
|---|
| The date and time (UTC) when the form was signed. |
Locked By
| Description |
|---|
|
The Username and ID of the user who locked the form. Note! In the case where the event date form is excluded from Viedoc Clinic, then in Viedoc Reports - Review Status report, the 'Locked by' will be marked as "N/A" (not applicable) since the form cannot be signed. |
Locked Date
| Description |
|---|
| The date and time (UTC) when the form was locked. |
Notes!
- Event Date is not included in the Form Status, but it is included in the Review Status. This means that N in the review status is greater than the number of triggered forms, for example, in the form status.
- For SDV, N is the total number of forms where SDV is possible. The N for other statuses counts the total number of forms as they apply to all forms.
- Forms that have partial items selected for SDV will display as blank, instead of Not required, in case SDV is pending.
