Medical coding
Medical coding
Medical coding
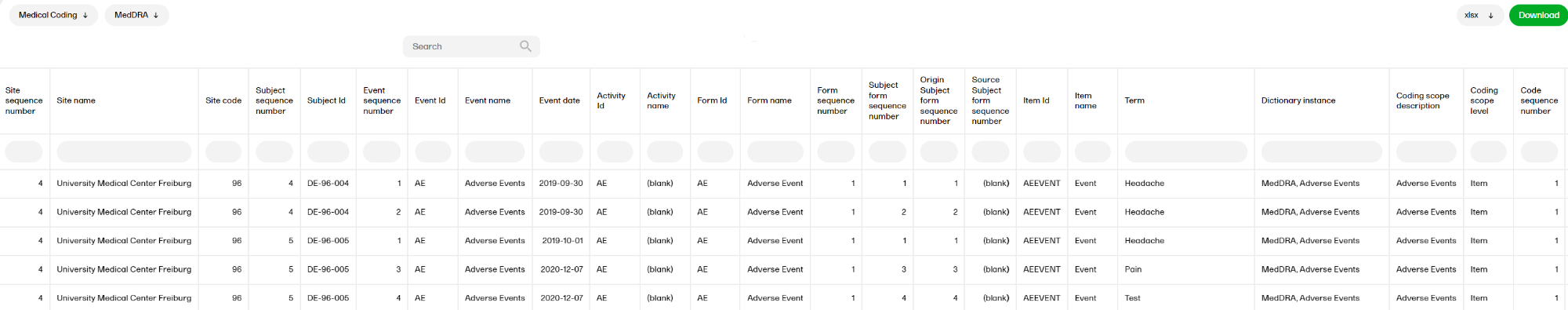
The Medical coding report shows the coded data in the study. You can select reports for the dictionaries WHODrug, MedDRA, MedDRA_J, ATC without DDD, and IDF - each containing dictionary-specific columns as well as the following columns:
- Site sequence number, Site Name, Site Code, Subject sequence number, Subject Id, Event sequence number, Event Id, Event name, Event date, Activity Id, Activity name, Form Id, Form name, Form sequence number, Subject form sequence number, Origin Subject form sequence number, Source Subject form sequence number, Item Id, Item name, Term Dictionary instance, Coding scope description, Coding scope level, Code sequence number
- Unique Term Report (UTR)... - these reports can be used as a quality and consistency check of the medical coding performed. Cases with identical terms that have been coded identically is merged into one row, and the number of cases is displayed. This means that one term that is coded in two different ways is split in two rows, and you will see the number of cases for each. This could indicate an issue with the coding selected.
You can also filter in the column with “coding discrepancy” to filter on these terms. The UTR can also simplify the review of the medical coding since this report is more compressed and easier to review than the standard coding export. - Note! The Medical coding data can be downloaded at all times (via a standard report, sample download, or custom reports) regardless of the user permission set for the role in Viedoc Designer.
# Coded terms
| Description |
|---|
| The number of coded terms. |
Coding discrepancy
| Description |
|---|
| This displays merged identical codes into a single row, showing the number of cases associated with the code. If there are two different codes, they are shown in separate rows with their respective case counts. |
Last term coded on
| Description |
|---|
| The last date coded for a particular term in UTC. |
# Coded terms approved
| Description |
|---|
| The number of coded terms that are approved. |
# Coded terms not approved
| Description |
|---|
| The number of coded terms that are not approved. |
