Handling booklets, AE reports and forms
Introduction
Viedoc PMS offers support for sending and receiving booklets back and forth between site and sponsor. For an overview of this process, see Overview of the submit-receive-return process.
In the submit-receive-return (Kaifu) process, the clinic user chooses when to share data with the sponsor and the sponsor-side user chooses when to receive the data. One of the important characteristics of this process is that the sponsor-side user does not have access to any data entered in a booklet, until the booklet has been submitted by the clinic, and a receive action has been actively performed by the user at the sponsor side.
Booklets can only be submitted to the sponsor when all events and forms in the booklet are completed.
There is an exception to this rule, which depends on your study configuration. It is possible that some forms (for example, but not limited to, Adverse Events) can be managed, that is, submitted/received/returned/reviewed/reported individually. When these forms are saved, a warning message is displayed and a Manage link is available. For more information, see Receiving booklets, AE reports and individual forms.
Note! As soon as an AE has been added, the system displays a warning message at the top of the booklet to facilitate the timely submission of AEs.
The status of the booklet is displayed on the subject Details page. You can select to display the complete history of submitting-receiving-returning the booklet by selecting Show history. You can select to display only the current status of the booklet by selecting Hide history.
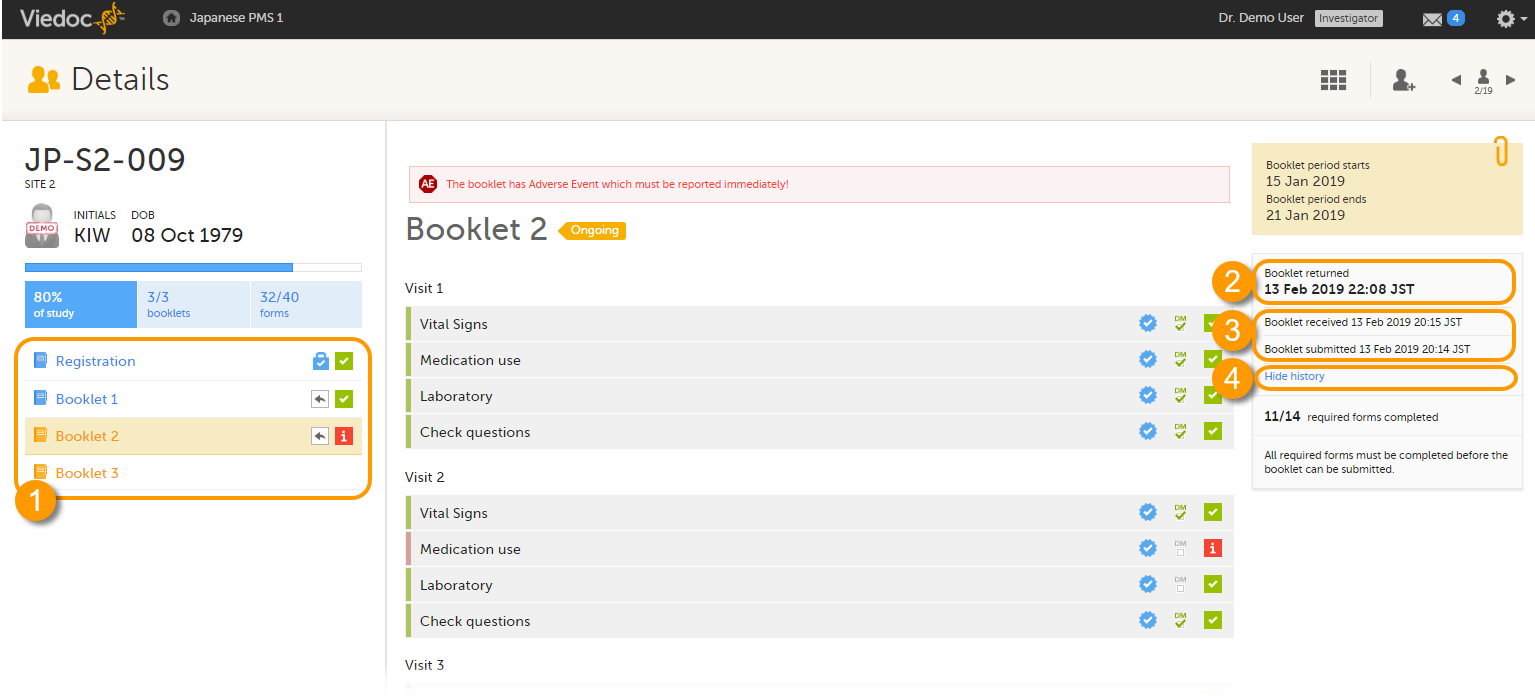
1. Select a booklet to work with
2. Current status of the selected booklet
3. History of the selected booklet
4. Show/hide history
Receiving booklets, AE reports, individual forms and linked forms
Overview
Forms can be received from the clinic side individually, depending on how your study is configured, you can:
- Receive only Adverse Events (AE) reports individually.
- Receive some of the other forms contained in a booklet individually, as well as AE forms, providing the AE form has been actively defined as an AE in the study design.
- Receive forms linked to another form in order to view complementary data added to a submitted form.
Note! Forms that are linked to other forms can be received individually or as part of a booklet submit. When a linking form (for example an AE) is linked to another form, (for example the Prior and Concomitant Medications form), and the AE form is submitted individually, a read-only copy of the linked form (Prior and Concomitant Medications) is received, if not already available on the sponsor side.
This applies even if the booklet is not yet completed.
There are different icons, warning messages and dialogs shown, depending on the your study configuration.
Icons and warning messages
The icons displayed on the form are as described below:
|
The forms marked as Adverse Events in your study are flagged with this icon. |
|
|
The forms that are NOT marked as Adverse Events in your study, but which can be managed individually are marked with this icon. |
The warning messages are as shown below:
Once receiving a form that was submitted individually, a warning message is shown. The warning message differs depending on the study configuration.

![]()
This applies even if the booklet has the status frozen.
Notes!
- If there is a new effective study design assigned to a site, and new forms have been selected to be submitted individually in that design version:
- Warning messages will only appear for received forms that are connected to the new forms added in the study design if the booklet does not have the status frozen.
Receiving booklets, AEs, other forms, and linked forms
To receive booklets, AE reports, and other forms that have been submitted from the clinic side:
| 1 |
Select Receive booklets. 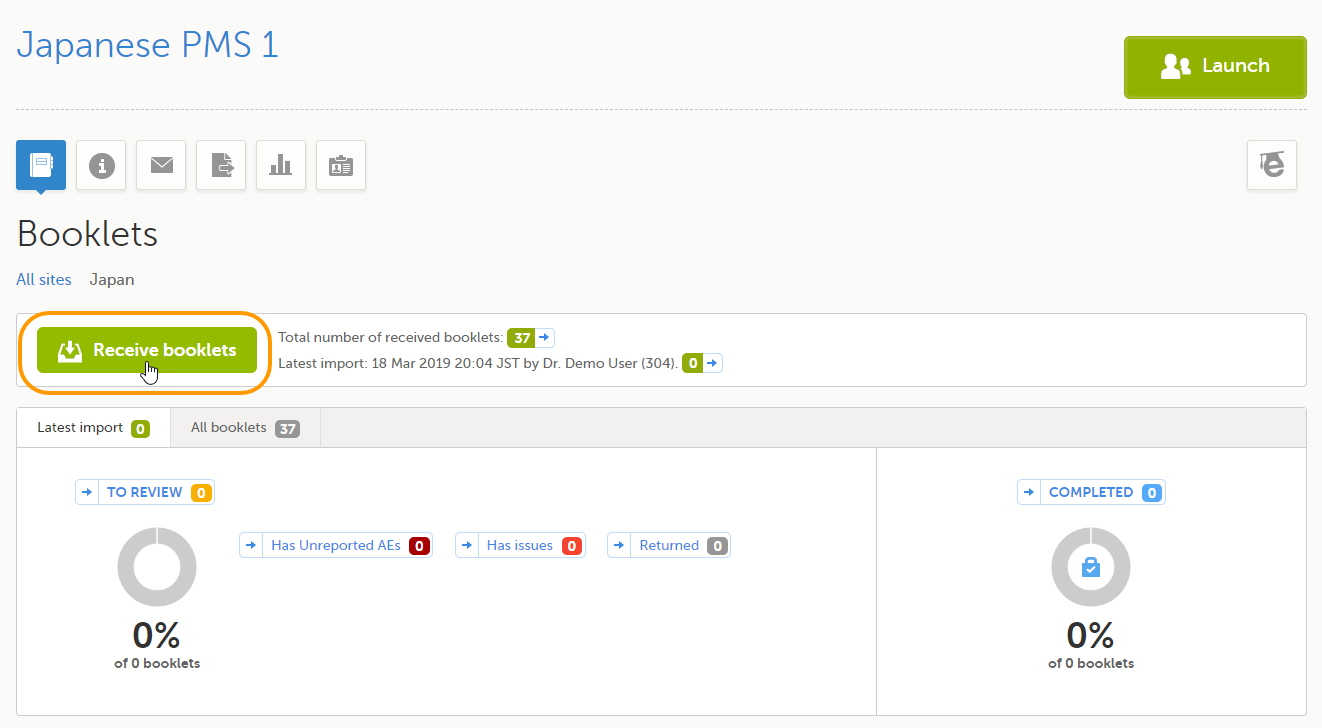
|
|
| 2 |
A window appears that asks you to confirm the receive action. 
|
|
| 3 |
After the booklet/AE report/form is confirmed as received, a Manage link is shown: 
On the relevant form, select Manage. |
|
| 4 |
A text appears. Depending on the study configuration and the received form, the text is as shown below: Manage Form 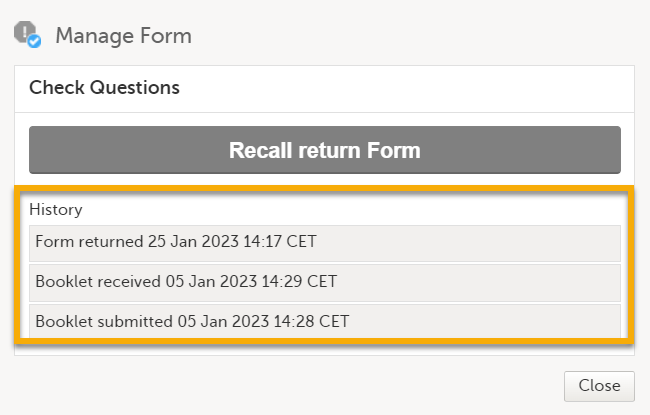
|
|
|
In History, the complete history of the form's submit-receive-return actions is shown. |
||
The information on the Latest import tab is updated with the booklet counts of the latest import.
Notes!
- The receive action takes site access into account. Thus, only the booklets submitted at sites to which the user has access will be received.
- You can always launch the study and enter the booklet overview without receiving booklets by selecting Launch, or by selecting one of the shortcuts to the booklet overview described in Booklet overview.
Viewing linked forms
You can view a linked form from the linking form by selecting any link item to open and read that form. In the example shown below, the Prior and Concomitant Medications form is the linked form. Closing the form returns you to the original (linking) form, in this example, the Adverse Event form.
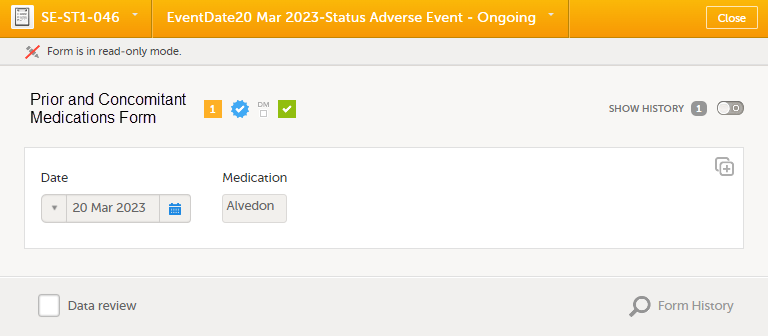
Note! If the linked form is not under control of the sponsor, that is, it is under clinic control, the linked form is available for the sponsor only as a read-only copy, and no Kaifu actions can be taken on the form (such as reviewing a form, returning a form, marking a form as reported, or adding queries, etc). When forms marked as AEs are received as linked form copies, they are not included in the AE forms count.
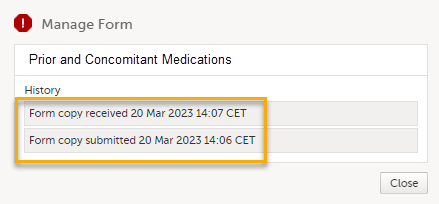
For a linked form that has been received as a read-only copy when a linking form was submitted individually, depending on the received linked form, the the complete history of the form's submit-receive-return actions is shown as below:
Handling booklets
Returning a booklet
To return a booklet, select Return booklet.
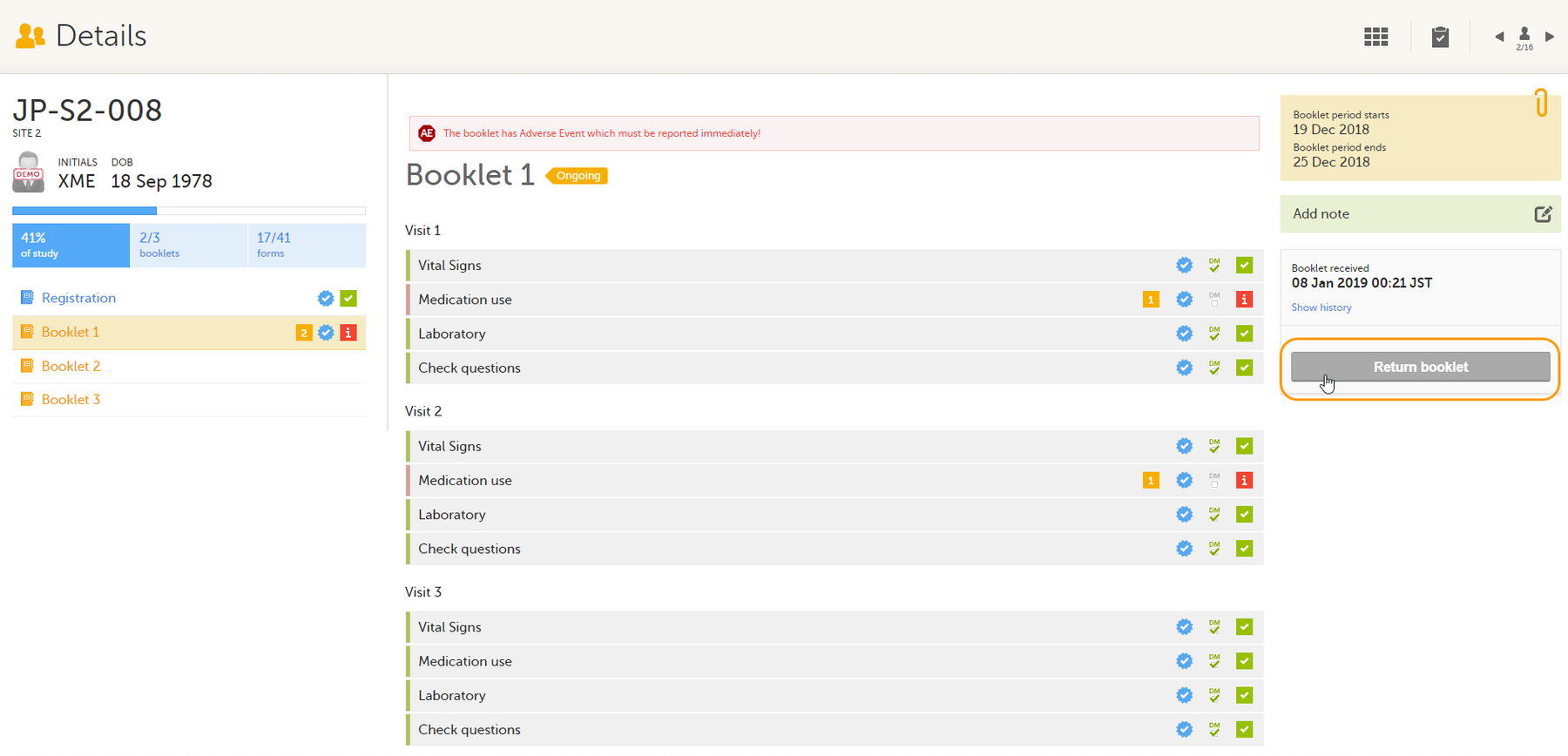
The booklet status changes into returned, and the date and time of the return action are displayed.
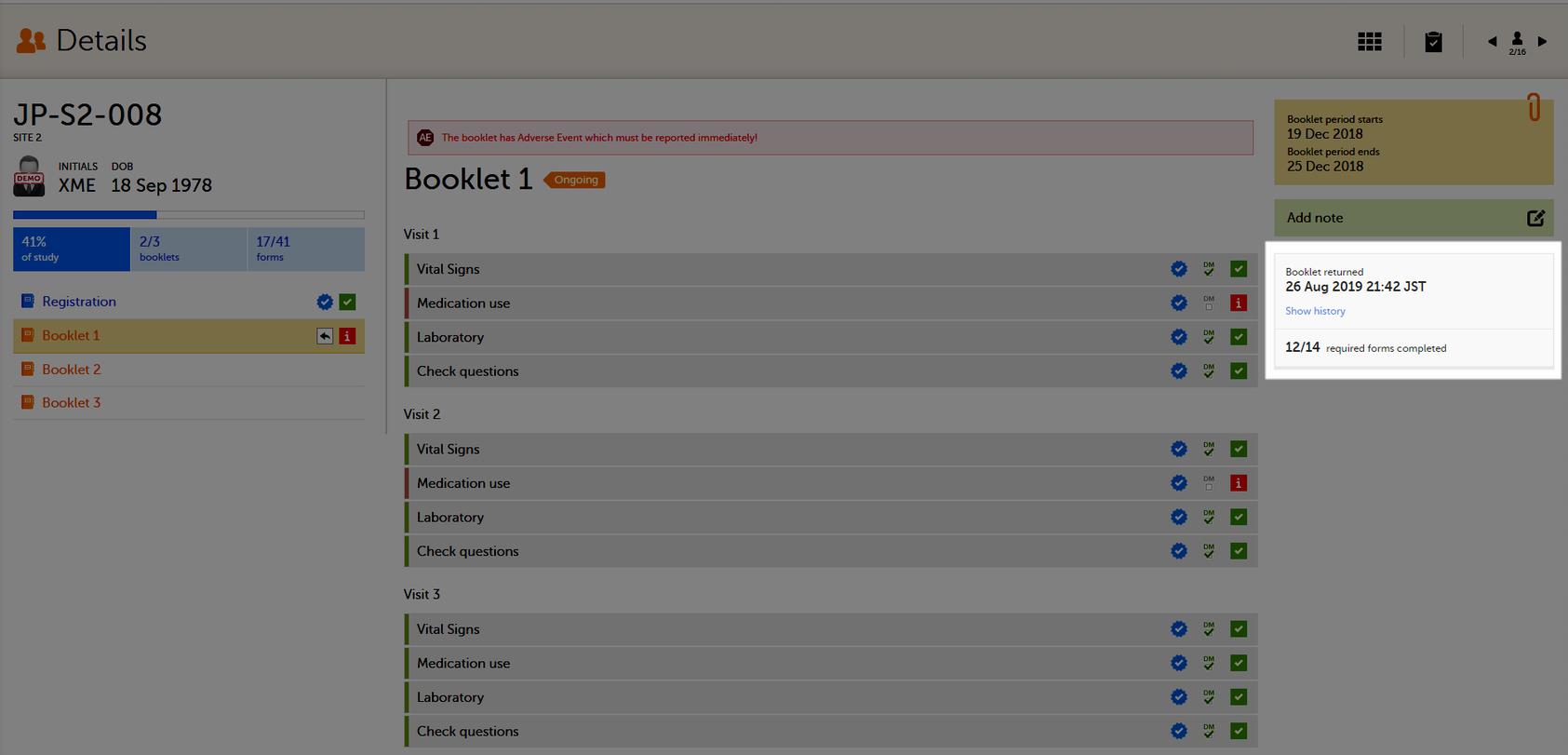
On the booklet overview, the booklet receives the following icon, indicating that the booklet has been returned.

Recalling a returned booklet
The recall function is available for users with the return booklet permission. The function is available when a booklet was returned to the Investigator but has not yet been changed/edited by the Investigator. After a sponsor performs a Recall return action, the booklet is brought back in control of the sponsor and it is no longer possible to edit the booklet on the clinic side.
To recall a booklet, select Recall returned booklet.

Note! If any data in the booklet is changed or added, or, if the booklet has already been recalled, the action is rejected.
After recalling a booklet, the following happens:
- The booklet status is changed to received.
- The action is tracked in an audit trail record telling who, when, and what was done. The audit trail record is visible on the booklet details page and is included in the booklet status export.
...which means that:
- All forms in a received booklet are locked for editing by the Investigator.
- Revision updates are queued instead of applied.
- The booklet is from now on visible and in control for the users who can receive booklets and for the users who can review booklets in the Details page.
Freezing a booklet
To freeze a booklet, select Freeze booklet.
Note! Booklets can only be frozen when all data in the booklet is completed, all data is reviewed and there are no open queries or other issues.
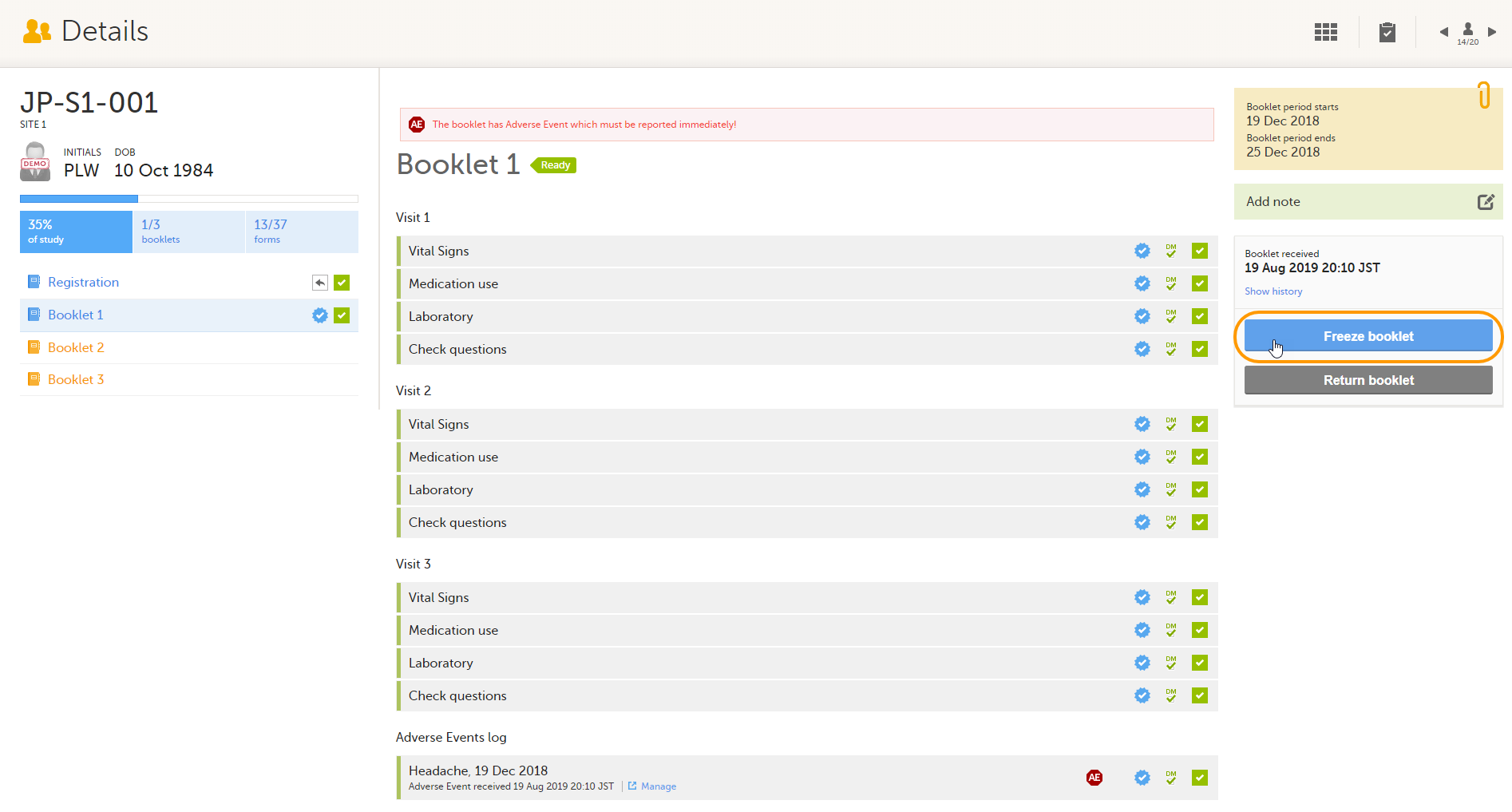 The booklet status changes into frozen, and the date and time of the freeze action are displayed.
The booklet status changes into frozen, and the date and time of the freeze action are displayed.
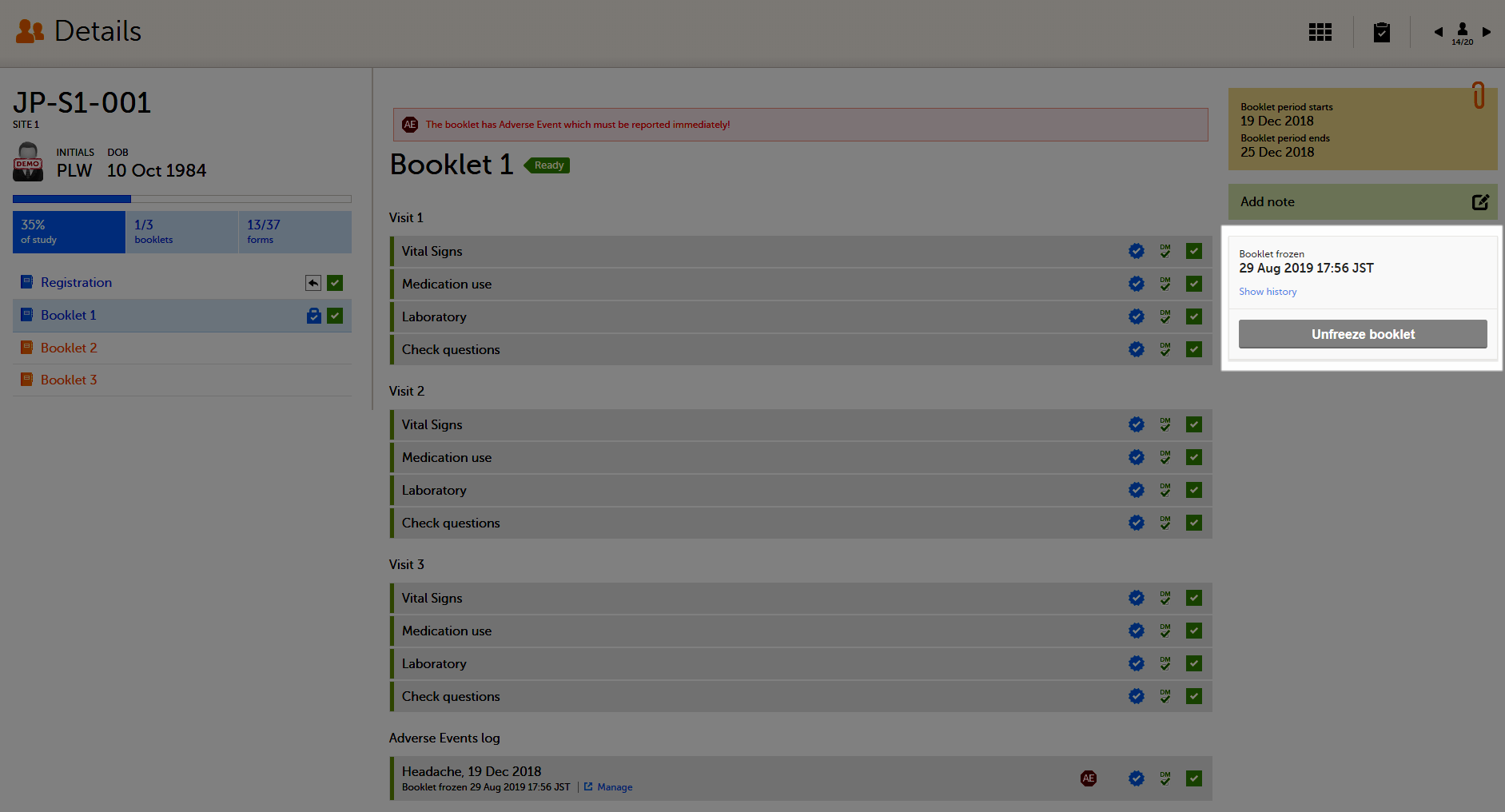
On the booklet overview, the booklet receives the following icon, indicating that the booklet has been frozen.

Handling AE reports and individual forms
Returning an AE report or an individually submitted form
Depending on your study configuration, AEs can be submitted separately as an AE report. Other individual forms and linked forms can be submitted separately, even if the booklet is not yet completed.
When an AE is registered, a warning message appears at the top of the booklet stating that The booklet has Adverse Event which must be reported immediately! The adverse event has the status Not reported, and a red AE icon appears on the form.
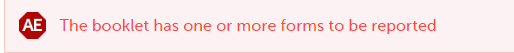
When another individual form is registered, a warning message appears at the top of the booklet stating that The booklet has one or more forms to be reported, and a red exclamation mark icon appears on the form.
Note!
- If the linking form has previously been received on the Sponsor side, a copy of the linked form will also be available on the Sponsor side.
- If the linking form is returned and deleted, and the booklet is then submitted, the form will be marked as deleted on the Sponsor side upon booklet receive and the linked copy will retain the same status.
To return an AE/individual form:
| 1 |
On the relevant form, in this example the Adverse Event form, select Manage. 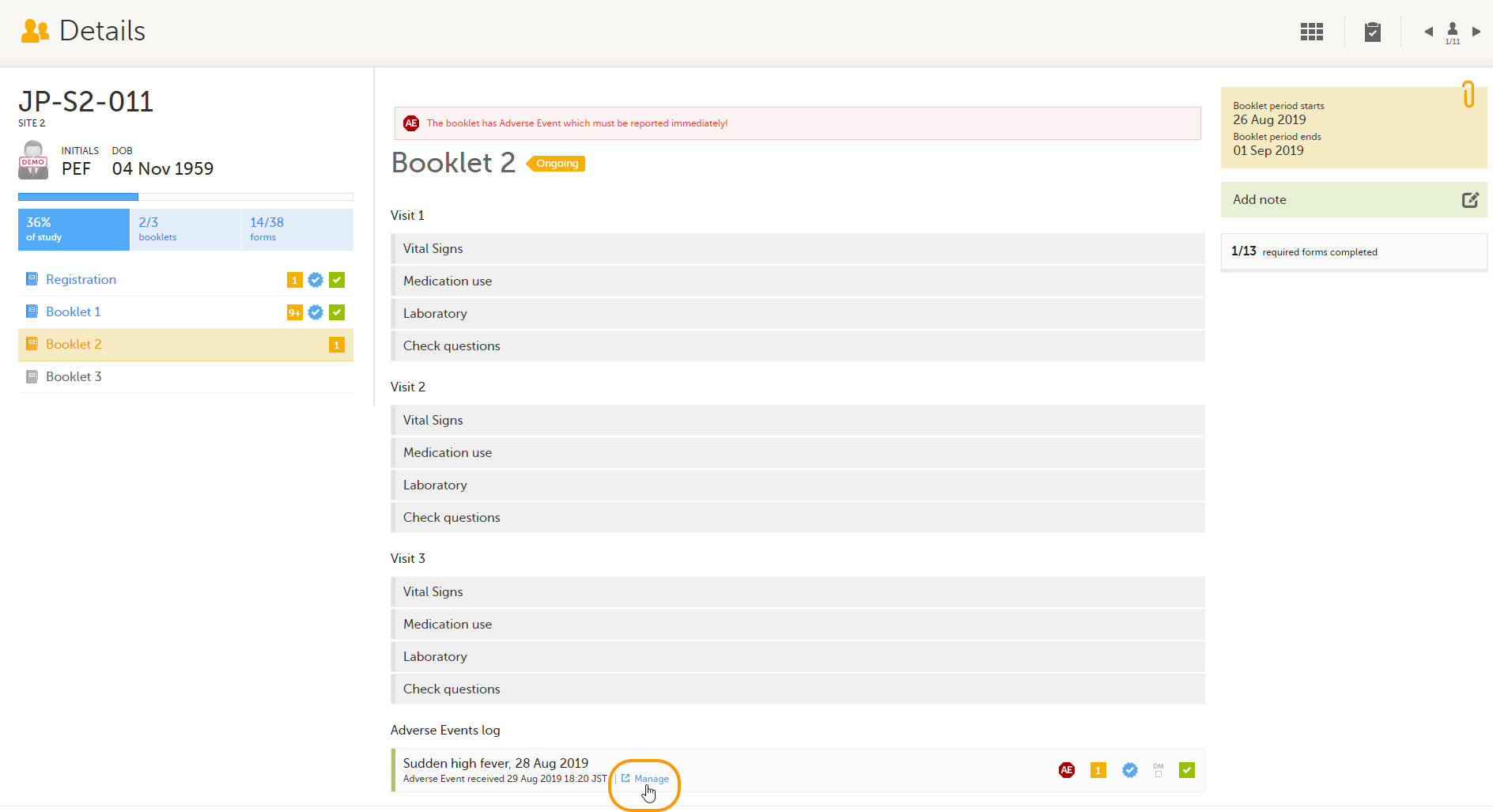
A window appears. |
|
| 2 |
Select Return Adverse Event. 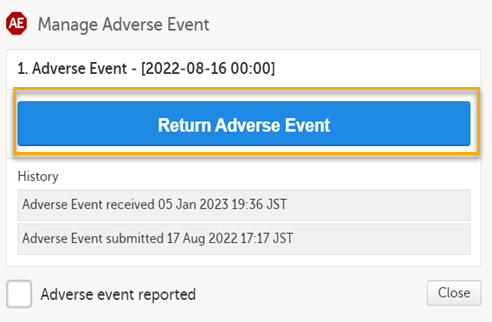
|
To return a form that was submitted individually, select Return Form. 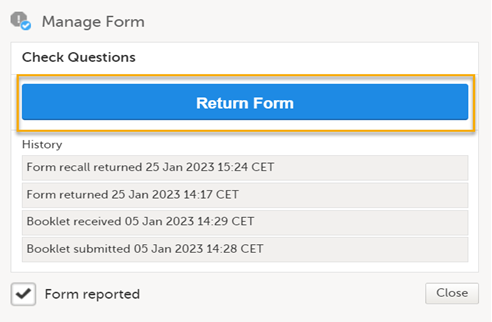
|
| 3 |
The status of the adverse event changes from Adverse Event received to Adverse Event returned + date of submission. 
The complete history of AE submission/recall/receive is shown in the history in the Manage Adverse Event window that appears upon selecting Manage. |
The status of the form changes from Form received to Form returned + date of submission. 
Note! Forms that are received as part of a booklet submit can only be returned if the form has been configured to be submitted individually in the effective study design. |
Recalling a returned AE report or an individual form.
The recall function is available for users with the return booklet permission. The function is available when an AE or an individually submitted form was returned to the Investigator but has not yet been changed/edited by the Investigator. After a sponsor return recall, the booklet is brought back in control of the sponsor and it is no longer possible to edit the booklet on the Clinic side.
To recall an AE or an individual form, select Recall returned adverse event/Recall return Form.
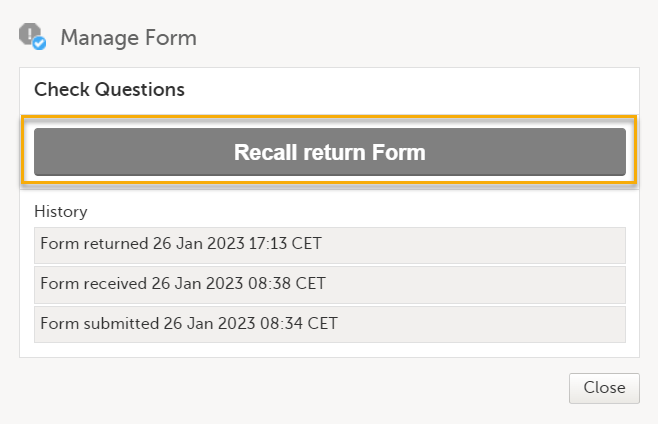
Note! If any data in the AE/individually submitted is changed or added, or, if the AE/individual form has already been recalled, the action is rejected.
After recalling an AE/individual, the following happens:
- The AE/other form is set back to received.
- The action is tracked in an audit trail record telling who, when, and what was done. The audit trail record is visible in the AE/Manage Form window.
...which means that:
- All forms in a received AE/individual form are locked for editing by the Investigator.
- Revision updates are queued instead of applied.
- The booklet/individual form is from now on visible and in control for the users who can receive booklets and for the users who can review booklets in the Details page.
Marking an AE/individual form as reported to authorities
Note! Forms that have been received on the sponsor side and do not have the status frozen can still be marked as reported to the authorities. Please note that an automatic notification is not sent to the authorities, this is to mark that the relevant authorities that should be notified have been notified. The purpose of this checkbox is to act as a review flag; for example, similarly to how a task is closed when performing SDV.
To mark an AE/individual form as reported:
| 1 |
Select Manage on the relevant form you want to report. The example below is an Adverse Event form. 
A window opens. |
|
| 2 |
Select the Adverse event reported and select Close. 
The status of the AE will change to reported, the icon on the AE form will change into a grey AE icon with checkmark, and the red warning text The booklet has Adverse Event which must be reported immediately! at the top of the page disappears. 
|
For other forms, select Form reported and select Close. 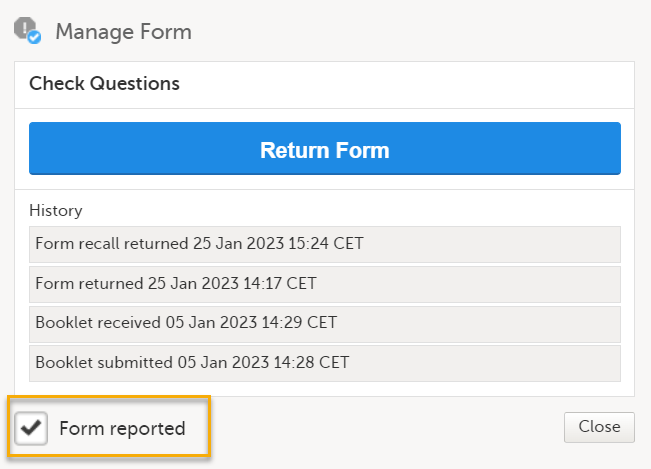
The status of the individually submitted form will change to reported, the icon on the Check Questions form will change into a grey exclamation mark with checkmark, and the red warning text The booklet has one or more forms to be reported at the top of the page disappears. Note! The text in the warning text may differ, depending on the study design. |
Note! Regarding the Adverse event reported checkbox, this checkbox can be selected and requests sent off for the form to be returned:
- If the form is submitted and the form is checked as reported on sponsor side, and returned to the clinic side, and then the form is subsequently edited on the clinic side and submitted, the checkbox is cleared.
- If the form is submitted and the form is checked as reported on sponsor side, and returned to the clinic side and then submitted to the sponsor side without any edits to the form, then the Adverse event reported checkbox is still selected, as no data has been changed.
- The status of the Adverse event reported checkbox is not reflected anywhere in the study data.
Handling deleted subjects
Receiving a deleted subject
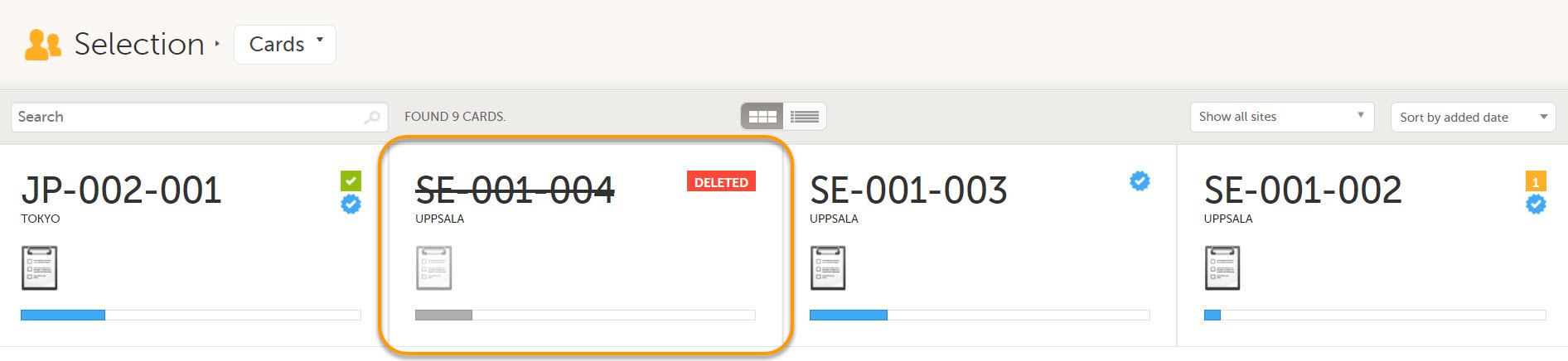
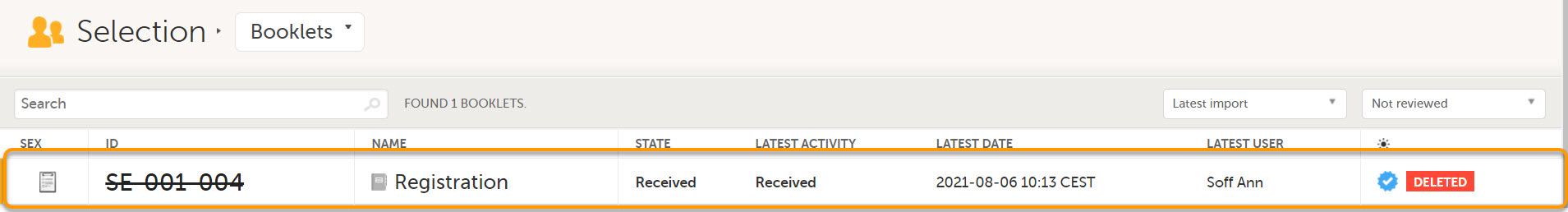
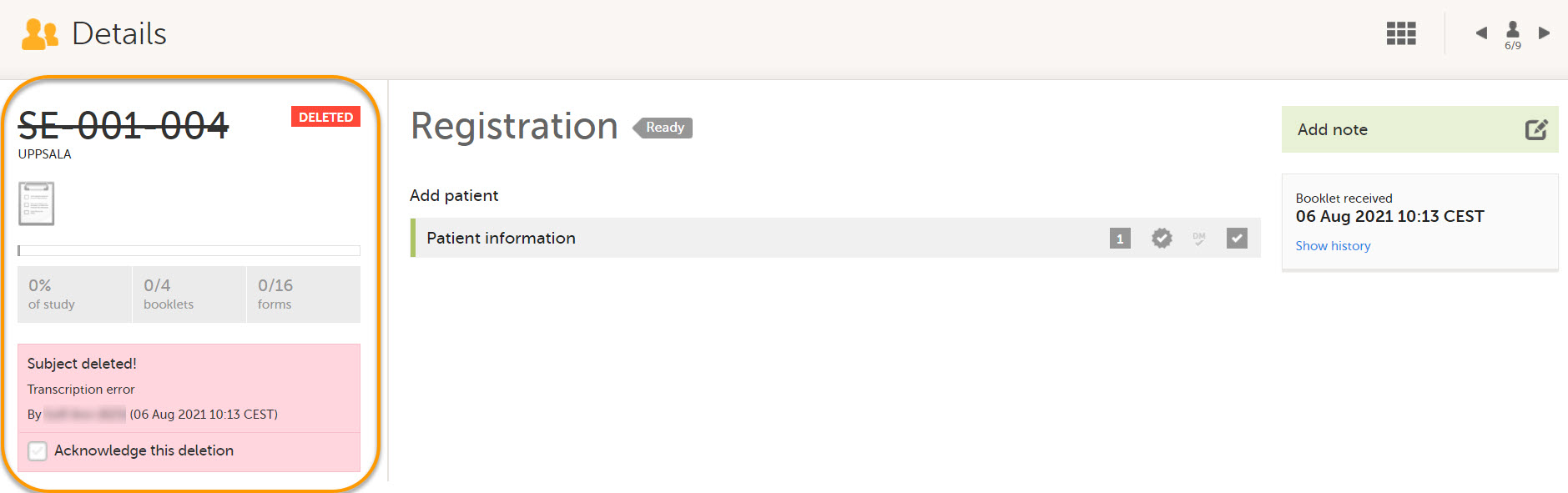
Deleted subjects are included in the receive action. Once data is received at the sponsor side, deleted subjects are displayed in the following places:
- Cards Selection page
- Booklet Selection page
- Subject Details page
Deleted subjects also appear in the Issues selection page if the deleted subjects had issues.
Acknowledging a deleted subject
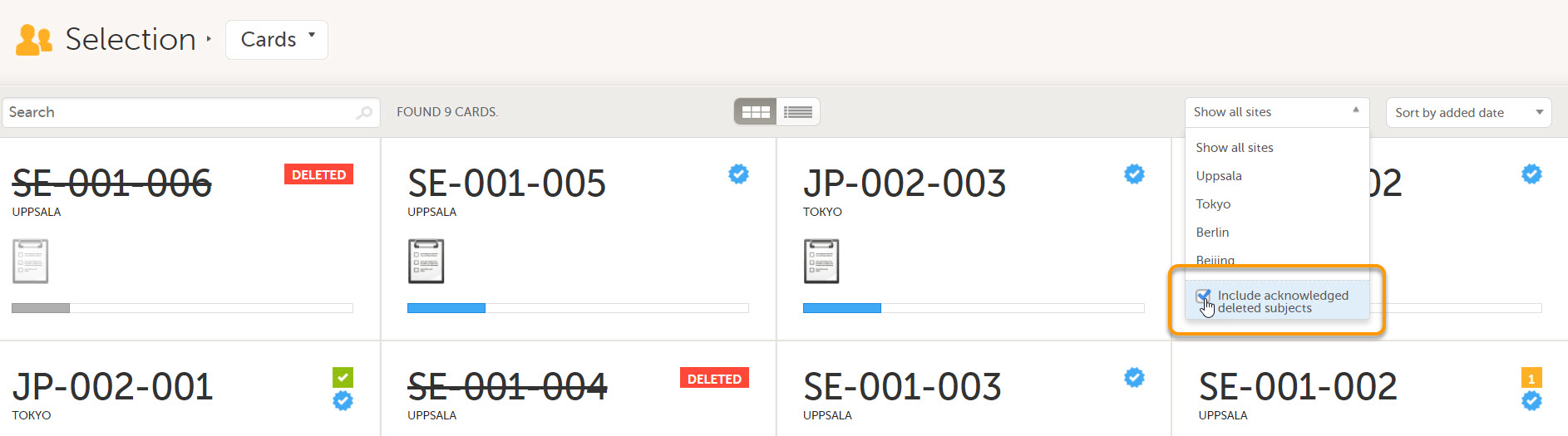
When deleted subjects have been received, they can be acknowledged by selecting Acknowledge this deletion on the subject Details page.
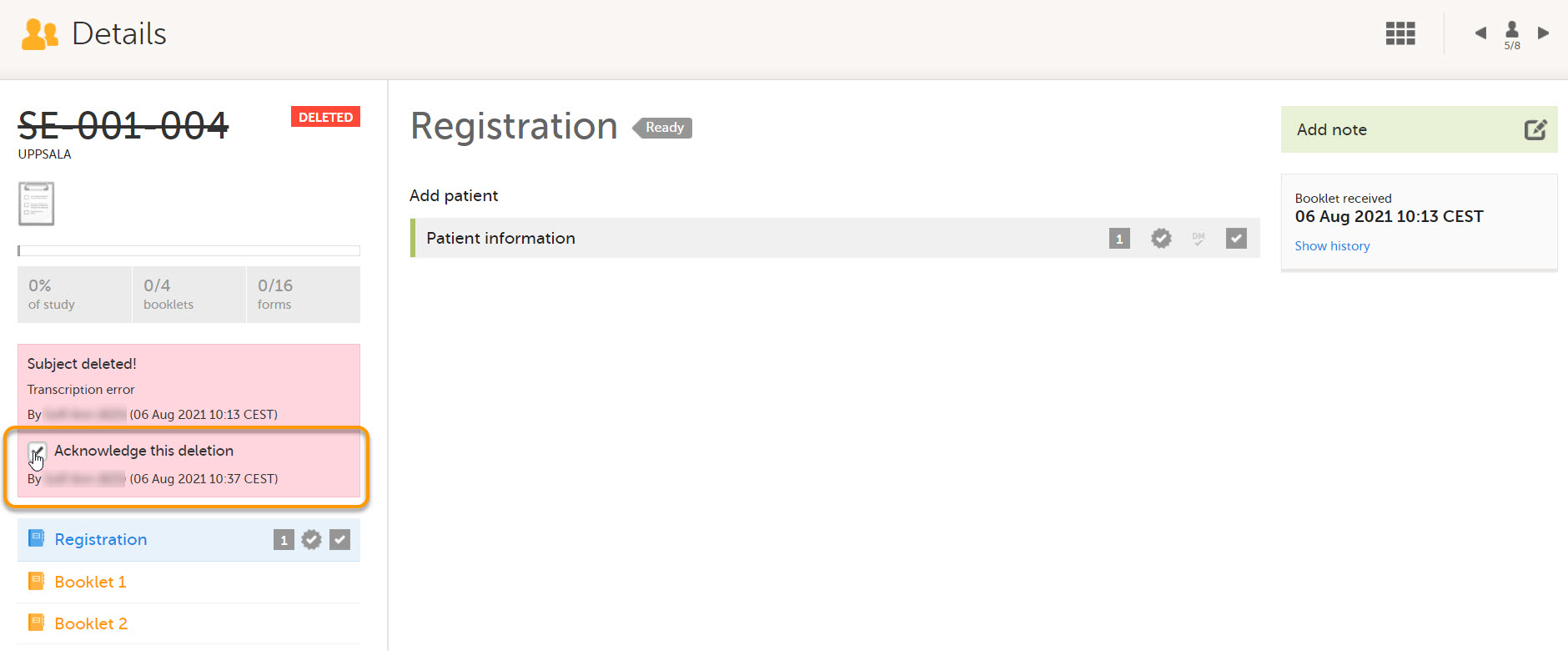
This action is recorded below the checkbox with information about who and when the deleted subject was acknowledged. The acknowledgment causes the deleted subjects to become hidden from the main views. To view acknowledged subjects, select Include acknowledged deleted subjects.
Updates to study design
If the study design is updated and configured so that forms can no longer be submitted individually, or if a new study design version is applied to a site, the following rules apply to the actions and messages for existing individually submitted forms:
- The existing individuallly submitted forms on the sponsor side are still visible on the Details page.
- Sponsor-side users can no longer perform Kaifu actions.
- Sponsor-side users can no longer select/deselect the form as reported/unreported in the Form reported checkbox in the Manage Form window.
- The Manage link is still available and can still be selected to show the complete history of the form's submit-receive-return actions.
Note! This only applies to individually submitted forms and not if the form was part of a booklet submission.
There are no warning messages displayed on the subject details page for that form.
Unreported Adverse Events
Forms that have been configured to be individually submitted and also are selected as AEs are included in the counters for Has unreported AEs on the sponsor side shown on the booklet overview page. See Booklet overview for more information.
NOTE! The Has unreported AEs counter is NOT updated when a form is received as a copy.
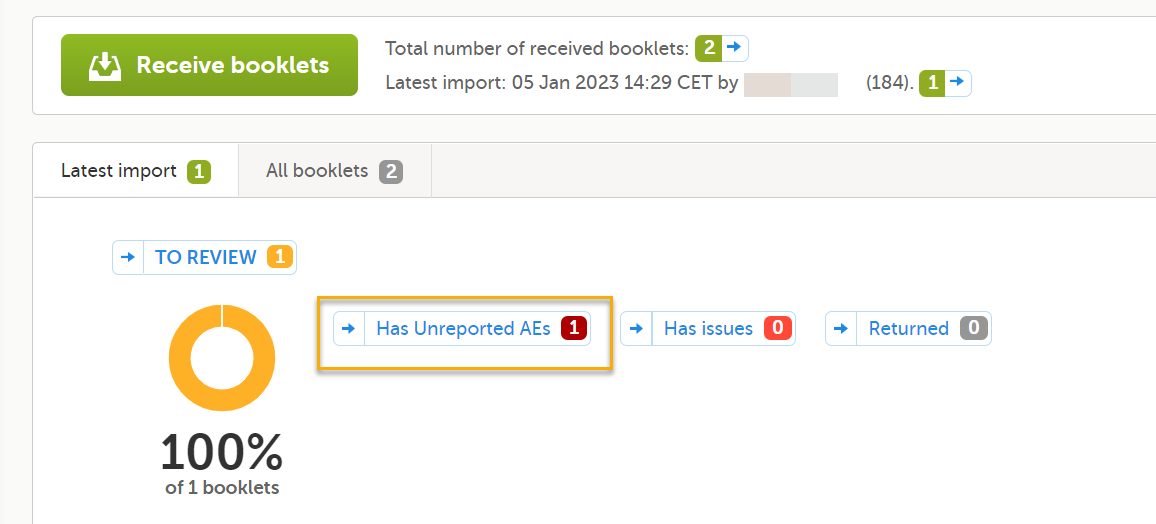
Unreported AE forms for studies where submitting individual forms is configured are counted when the following conditions apply:
- Select as Adverse Event has been selected for the form in the study design.
- The form on the sponsor side is not marked as reported.
- The Booklets selection page status is filtered by Has AE.