Design revision impact analysis
Before applying a new design revision, Admin users with the system role Design Impact Analyst can use the design revision impact analysis tool to perform an impact analysis. The analysis shows the number of existing form instances per site that will require confirmation by site staff, regardless of who created the revision.
Important! It is recommended that this revision impact analysis is used before applying any revision.
The design revision impact analysis report
The Design Impact Analyst can generate, regenerate, and download the Excel report.
Generating the Excel report
To generate the Excel report:
| 1 | In Viedoc Admin, select your study. |
| 2 |
Select Edit in the Study design section. 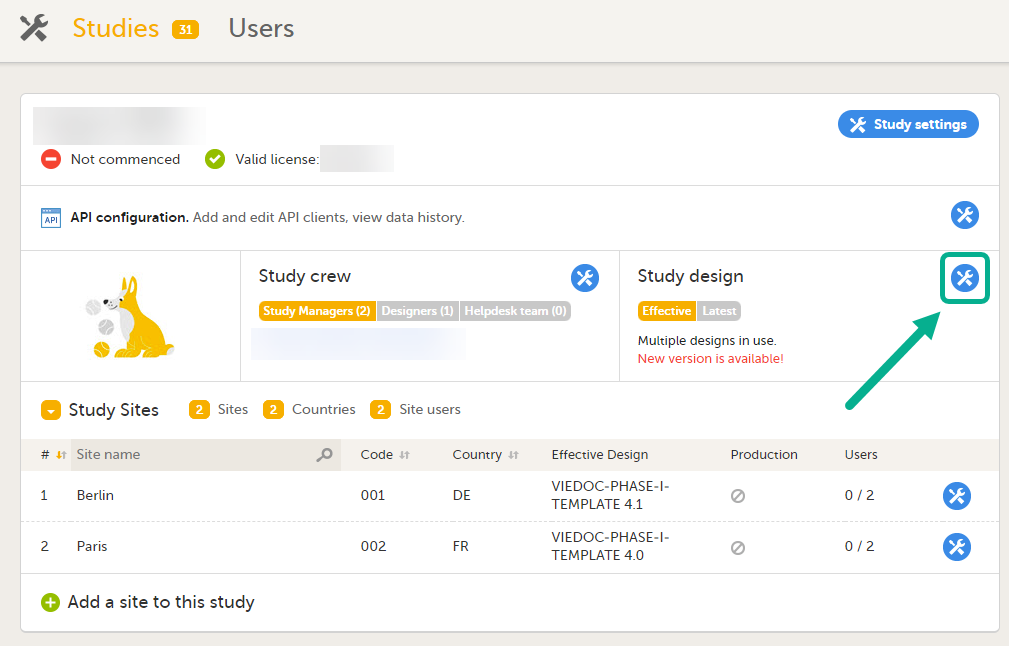
|
| 3 |
In the Design settings window, open the tab Apply revision. 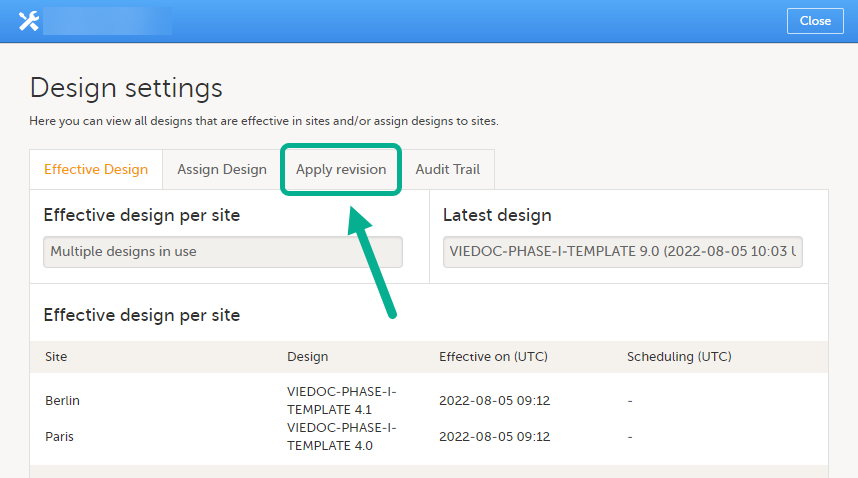
|
| 4 |
On the tab Apply revision, select a design revision in the dropdown menu. 
|
| 5 | Select Continue to go to the next step. |
| 6 | Select the sites where the revision will be applied. |
| 7 | Select Continue to go to the next step. |
| 8 |
In step 3, you'll see a summary of the revision. The table includes a column called Req confirmation, which is the number of existing form instances that will require confirmation from site staff. The time of the latest performed impact analysis per site is shown in the table. 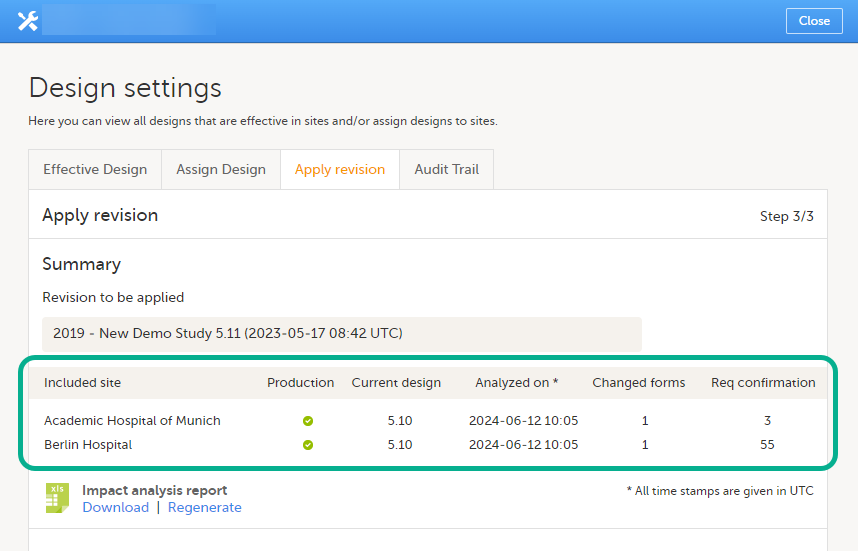
|
| 9 |
If this is the first impact analysis, you can select to Generate the report. Then select Download. For subsequent sessions, you can select Download or Regenerate. 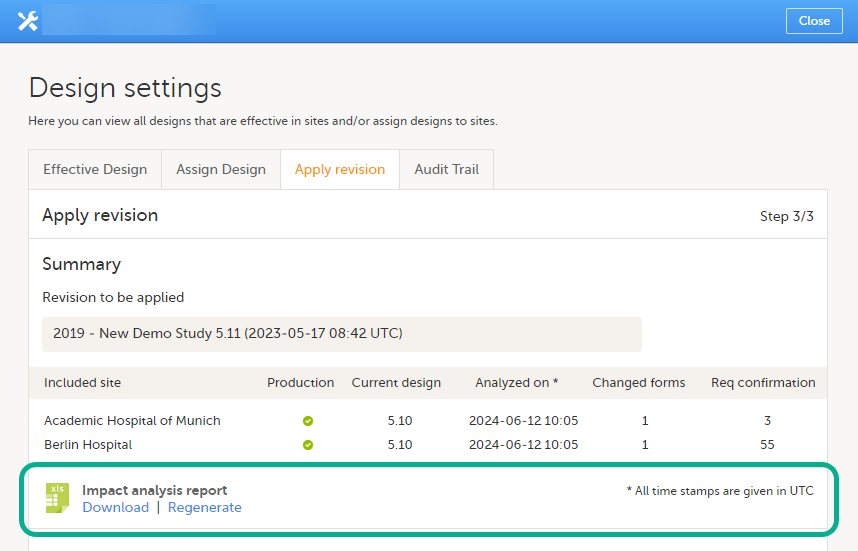
|
The contents of the Excel report
The Excel report contains these sheets:
- Summary - general information about the report
- Production - the forms for subjects at the analyzed production sites
- Training - the forms for subjects at the analyzed training sites
The Summary sheet shows the number of forms that will potentially lose their status regarding these parameters:
- Signature
- SDV
- Clinical Review
- Data Review
Note! By default, the contents of the Excel sheets Production and Training are filtered to show only the rows that have the value Yes in the column Req confirmation.
The Production and Training sheets contain these columns:
| Column | Description |
|---|---|
| Site Code | The site code, as defined for the study |
| Site Name | The site name, as defined for the study |
| Current Design | The version and revision number of the currently used design for the site |
| Revision being analyzed | The version and revision number of the design revision that is being analyzed |
| Subject Key | The subject key |
| StudyEventDefId | The ID of the study event |
| StudyEventRepeatKey | The number of repeats of the study event |
| FormDefId | The form ID |
| FormRepeatKey | The number of repeats of the form |
| ActivityDefId | The activity ID |
| FormEditStateLocked |
Yes - if the form was locked for edit when the report was generated No - if the form was NOT locked for edit when the report was generated |
| Req confirmation |
Yes - if the form instance will require a confirmation by site staff after the revision has been applied No - if the form instance will NOT require a confirmation by site staff after the revision has been applied |
| Signed |
Yes - if the form instance is signed No - if the form instance is NOT signed |
| SDV |
Yes - if the form instance has been SDVd No - if the form instance has NOT been SDVd |
| ClinicalReview |
Yes - if the form instance has undergone a clinical review No - if the form instance has NOT undergone a clinical review |
| DataReview |
Yes - if the form instance has undergone a data review No - if the form instance has NOT undergone a data review |
| PMS side |
Note! This column is only available for PMS studies. Clinic - if the form belongs to the Clinic side of the PMS study. Forms that are submitted but not yet received belong to the Clinic side. Sponsor - if the form belongs to the Sponsor side of the PMS study |
Unblinding
It is important to understand that the revision impact analysis provides a lot of details about the upcoming revision, and it might even be unblinding in certain circumstances.
If a study design uses role-based visibility for items that could reveal the subject's treatment, and the mere presence of the item is unblinding, the revision impact analysis could reveal which treatment the subject is on.
For this reason, the permission to view and generate the revision impact analysis is isolated to a dedicated user role, and we recommend caution before you invite a user with this role.
Example 1
A CRF design uses a role called treating investigator and another called evaluating investigator. The treatment is blinded to all users in the study except for the treating investigator.
The RTSM settings for the design uses a non-blinded outcome to display the treatment, but this is only visible to the treating investigator.
Subjects are assigned to treatment X or treatment Y.
Based on the assigned treatment, in the randomization form, the form Drug Administration is triggered. This form is filled in by the treating investigator and hidden to all other users because this is also revealing the treatment of the subject.
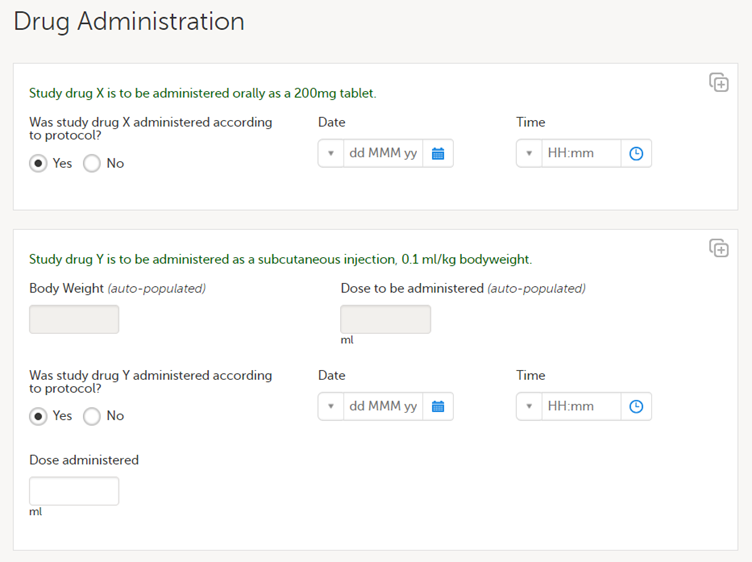
The first item group is only displayed to subjects assigned to treatment X, and the second item group is only displayed when the subject is assigned to treatment Y.
Let's assume that there are complaints on this CRF design, and the second item group is changed in a revision by changing the label of the final item from Dose administered to Actual dose administered for clarity. The revision impact analysis will then show which forms, and for which subjects, forms are requiring a manual upgrade - this would be all subjects that have been assigned to treatment Y. This is where the revision impact analysis would be unblinding.
In a scenario like this, we recommend that an unblinded user in the study team is invited with the Design Impact Analyst role to avoid unblinding to other members of the study team.
Note! If a designer has access as a Design Impact Analyst, this user could theoretically identify the treatment of each subject by creating a revision with changes to sensitive items (as described above), with the sole purpose to see the impact in the impact analysis report, and afterwards deleting the revision. For this reason, we recommend that the Design Impact Analyst role is not given to a designer when the CRF is designed according to the example above.
Example 2
A similar study is using the same approach with the roles treating investigator and evaluating investigator. The difference is that, in this CRF design, dosing details for treatment X and treatment Y are captured in the same form, using the same item. The difference between subjects assigned to the different treatments will be the values in the items rather than the presence of certain items.
If a change to a label is done in a revision, the revision impact analysis will NOT be unblinding, because all randomized subjects are expected to have the same items.
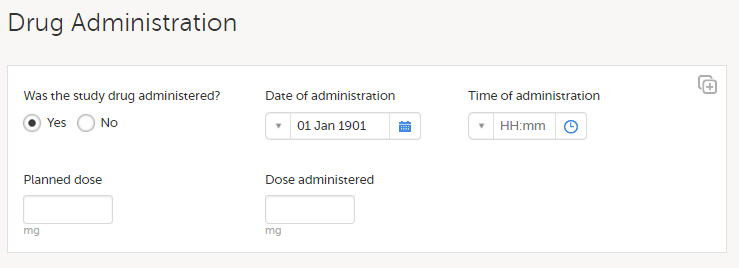
In a scenario like this, any user of the study team could be invited with the Design Impact Analyst role, as this will not risk unblinding other members of the study team. The recommendation is to invite users that would be responsible for applying the revision to this role so they can see the impact before they apply a revision.
Thus, if a study design uses role-based visibility for items that could reveal the subject's treatment, and the mere presence of the item is unblinding, the revision impact analysis could reveal which treatment the subject is on.
