Overview of Viedoc Admin
This section provides an overview of Viedoc Admin. It summarizes the main settings that can be configured in Viedoc Admin.
Introduction
Viedoc Admin is the starting point for every new Viedoc project. Viedoc Admin is the application where you can manage the administrative aspects of a study. The following actions can be performed in Viedoc Admin:
- Add a new study
- Manage user accounts
- Invite users to system roles and clinical roles
- Add study sites
- Assign designs to study sites
- Manage general study settings and fill out study details
- Manage randomization lists
- Upload coding dictionaries and create coding instances
- Manage reference data sources
- Configure the Application Programming Interface (API)
Access to Viedoc Admin is granted by either the Organization Administrator or the Study Manager.
Organization overview
For the Organization Administrator, the organization overview is the first page that is shown upon accessing Viedoc Admin.
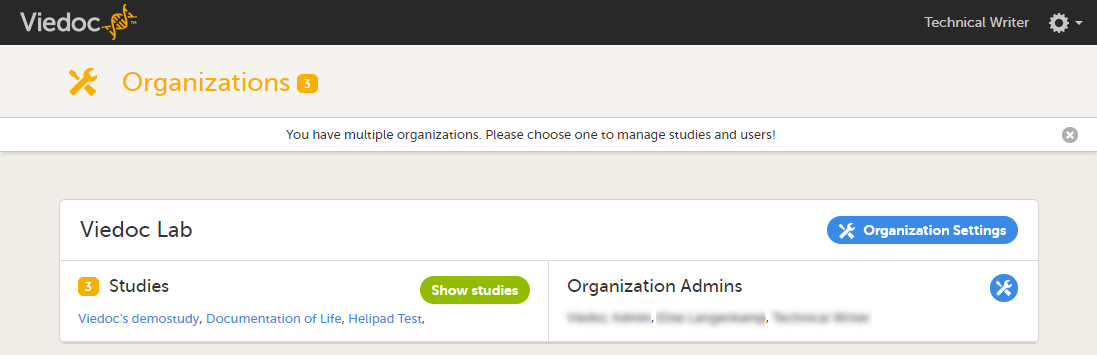
On the organization overview, you can:
- Edit the Organization Settings, for example update the contact details for the organization and configure single sign-on.
- View or access all studies within the organization. Click Show studies to view a detailed list of all studies, or click on the name of a study to directly access the study.
- View a list of all Organization Administrators, and invite users to the role of Organization Administrator. Click the toolbox icon to open the organization administrators dialog. For more information about how to assign organization administrators, see Managing users (Org Admin).
Study overview
For all users that are not Organization Administrator, the study overview is the first page that is shown upon accessing Viedoc Admin. This page lists all studies in which the user has a system role.
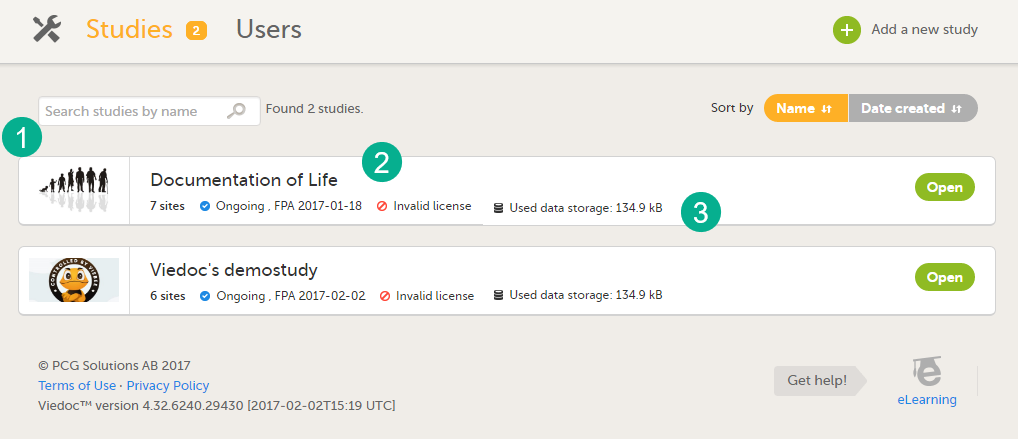
For each study, the following information is displayed:
1. The logo of the study
2. The name of the study
3. Some study details are:
- The total number of production and training sites
- The study Status
- The date of first patient added (FPA) (only for production sites)
- The status of the study license number, if a valid license exists
- Used data storage
Study status
This section explains Study Status.
A study can have these statuses:
- Not commenced
- Ongoing
- Locked by (the name of the user who locked the study)
- Study delete requested by (the name of the user who requested the study delete)
- Study delete confirmed by (the name of the user who confirmed the study deletion) - this is only visible for the Organization Administrator. For other users, the study disappears from the Studies overview once the study deletion is confirmed by the Organization Administrator.
Note! The study status will change from Not commenced to Ongoing when the first production site is added. A study license is required to make that change.
Used data storage
Your used data storage keeps track of the amount of data used by the documents added in Admin, as well as the files uploaded in eCRF.
Open a study
To open a study and access the study details page, click the study. You can search for a study by entering the study name in the search field. You can sort the studies by study name or by the date when the study was created.
Note! A study needs to have a valid license to be taken into production. For more information about the study license, see the chapter about licensing in Overview of Viedoc. For more information about how to take a study live, see Adding a new study.
The study details page
The study details page is the first page that is shown upon accessing a study. On the study details page, you can interact with the settings in the following ways (see image):
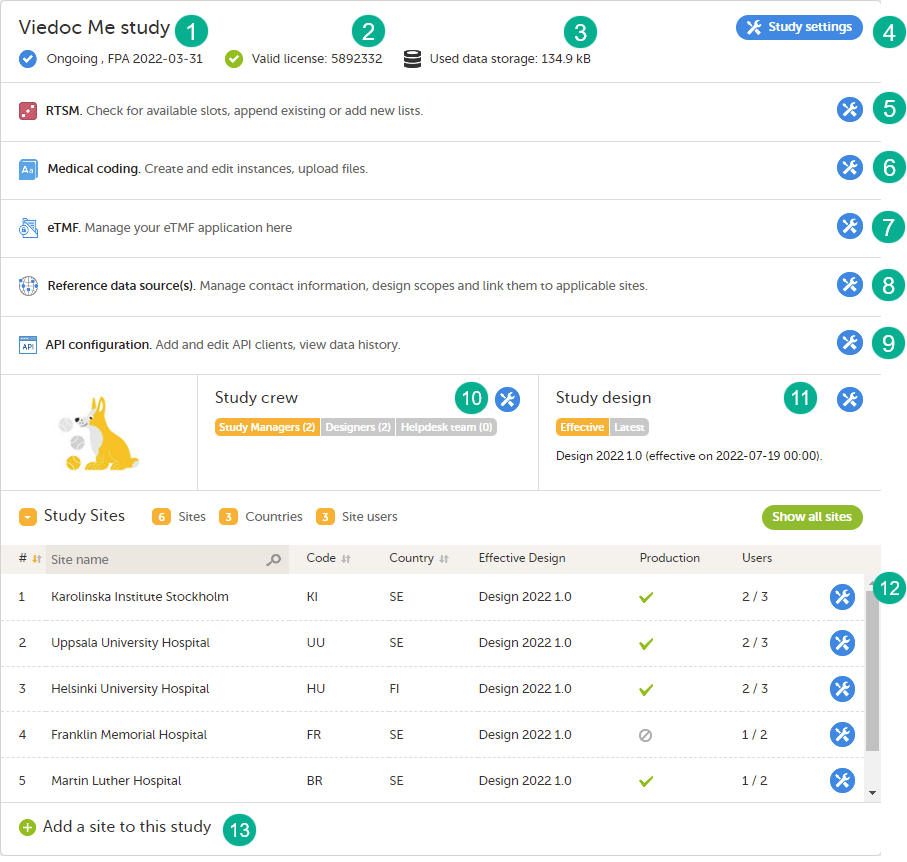
1. FPA
2. License status
3. Data storage
4. Edit the general study settings, see General study settings.
5. Manage the reference data sources, see Managing reference data sources.
6. Upload and manage medical coding dictionaries, see Managing medical coding dictionaries.
7. Manage the eTMF, see Quick guide for setting up Viedoc TMF
8. Manage the reference data sources, see Managing reference data sources.
9. Configure the API, see Viedoc WCF API, API configuration, and Viedoc Data Import Application.
10. Manage the study crew, see Managing users (Org Admin) and Managing users (STM and SIM).
11. Apply study design versions and revisions, see Assigning a study design.
12. Edit the study site settings and invite users to the study site, see Managing study sites and Managing users (STM and SIM).
13. Add study sites, see Managing study sites.
